Wednesday, March 8, 2017, the Office of the United States Trade Representative (USTR) will hold the first Special 301 Hearing of the Trump administration. This year, USTR received 63 submissions in advance fo the hearing from governments, civil society organizations, and industry groups.
This blog post pulls out interesting selections from the various submissions, and includes as attachments the submissions of selected organizations.
Civil Society
Knowledge Ecology International (KEI)‘s comments for this year are available here. This year, KEI focused on the misuse of employment data by industry groups to argue for expanded IP protection, particularly a 2016 USPTO study that misleadingly bolsters employment attributable to IP-intensive industries by including “trademark-intensive industries,” such as grocery stores, in their count. KEI also recommended that the Trump administration improve transparency in trade negotiations and explore the progressive delinkage of research and development costs from drug prices, through, for example, the expansion of the orphan drug tax credit.
Public Citizen criticized USTR for its past attacks on the use of international public health flexibilities and rights — especially the use of compulsory licenses — in past Special 301 reports. To remedy the situation, PC asked USTR to adopt principles to guide the Special 301 review process, and in particular to omit “expressed or implied references to countries’ public interest practices that comply with international treaty obligations,” including actions taken in compliance with the TRIPS agreement. In particular, PC asked USTR to stop criticizing countries for not adopting U.S. policy preferences, even when those countries had no obligation to do so. “Pharmaceutical or other public policy disagreements should never land a country on the Priority Watch List” (emphasis in original), Public Citizen wrote.
Michael Palmedo of the American University Washington College of Law Program on Information Justice and Intellectual Property emphasized the benefit of “open, flexible and general limitations to copyright law” for U.S. businesses, and asked USTR to focus on “countries that are moving to adopt more flexible copyright practices in its ‘Best Practices.’”
Noting that the “fundamental rights” of Colombian citizens should take priority over U.S. trade interests, Fundación Karisma, Ifarma, and Misión Salud submitted joint comments highlighting the disconnect between USTR’s stated respect for the use of compulsory licenses in past Special 301 reports and its attempt to block Colombia’s compulsory license on the expensive leukemia drug Glivec. The groups also argued that the “unilateral adjudication of trade disputes through the Special 301” violates the Dispute Settlement Understanding of the WTO.
Doctors Without Borders/Médecins Sans Frontières (MSF) submitted testimony urging USTR to “respect legal safeguards such as India’s strict patentability criteria; its right to issue compulsory licenses when deemed necessary in the interest of ensuring the right to health; and a balanced approach in the enforcement of private IP protections.” MSF also also noted their concerns with pressure on Colombia over their proposed compulsory license on imatinib, stating that no country “should be penalized” for protecting public health.
Industry
BSA | The Software Alliance
The Software Alliance (BSA) asked USTR to put Argentina, Chile, China, India, Indonesia, Russia, Ukraine, and Vietnam on the Priority Watch List and Brazil, Greece, Kazakhstan, Korea, Mexico, Nigeria Romania, Thailand, and Turkey on the Watch List. They also noted Spain as a country and the EU as a region of concern.
BSA voiced concerns with issues related barriers to cross-border data flows, procurement discrimination, security of software products, technological standards that favor local companies, and protection of software patents and trade secrets. BSA also criticized the unlicensed use of software by governments and large enterprises.
Biotechnology Industry Organization (BIO)
The Biotechnology Industry Organization (BIO) touted the economic, public health, and employment benefits of the biotechnology industry, citing industry-funded research the development of cures for HCV, and the falling rate of cancer deaths, amongst various other indicators. BIO identified “practices that undermine biotechnology innovation,” including patent backlogs, the use of compulsory licenses, restrictive patentability criteria, and regulatory data protection failures.
As is their normal practice, BIO also recommended a list of countries to be included on the various Special 301 lists. Of particular interest are BIO’s criticisms of Colombia for its attempt to issue a compulsory license on the leukemia drug imatinib (more here: /colombia), Canada over its rare use of injunctions to block patent infringement, India over section 3(d) of its Patents Act and the grant of a compulsory license on Nexavar, and Thailand for the use of compulsory licenses.
* = also requested Out of Cycle Review
Priority Watch List
Algeria
- No regulatory data protection for pharmaceuticals.
- Prohibition on imports of pharmaceuticals that are locally manufactured.
- Linking pricing and reimbursement to marketing authorization.
Argentina
- Patent backlogs.
- No patent term extension.
- Restrictive patentability criteria.
- No regulatory data protection for pharmaceuticals.
- Proposed bill to limit patentability of seeds.
Brazil
- Restrictive patentability criteria.
- Patent backlog.
- Review by the drug regulatory authority of all pharmaceutical patents prior to issue.
- Enforcement and royalty payments.
Canada*
- The “promise” doctrine and utility criteria.
- Regulatory process and forum for resolving patent infringement cases is biased towards generic manufacturers.
- Rare use of injunctive relief in infringement cases.
- Lack of patent term restoration.
- CETA implementation.
- Practices of Canadian online pharmacies.
- Use of cost effectiveness analysis to evaluate orphan drugs.
- Use of price controls by the Patented Medicines Review Board.
Chile
- Restrictive patentability criteria.
- Lack of adequate test data protection for data required for submissions for marketing authorization.
- Insufficient patent term restoration.
- Failure to comply with requirements in U.S. Chile FTA to not grant marketing approval to generics prior to the expiration of a patent.
China
- Restrictive patentability criteria and exclusion of protection for plant varieties.
- Lack of patent term extension.
- Lack of patents on genetic resources.
- Compulsory licensing provisions related to inadequate working of patents in China.
- Requirement to sell a product in China prior to filing an action for infringement.
- Unenforced regulatory data protection for foreign companies.
- Proposal to require that manufacturers agree to a price ceiling in China of a price no higher than the manufacturer’s home country or China’s surrounding markets.
- Counterfeiting.
Colombia*
- Compulsory license on imatinib.
- Patentability criteria on biological material and the genome.
- Patent infringement adjudication.
Ecuador
- Policies on compulsory licenses.
- Ineffective regulatory data protection.
- Undermining of trademark rights.
- Patent application fee increases.
India*
- Section 3(d) of the Indian Patents Act and other patentability criteria.
- Disclosure of source and geographical origin of biological materials used to make a patented invention.
- Exclusion of plants from patent protection.
- Lack of “meaningful” regulatory data protection.
- Lack of mechanisms to prevent generics from entering the market.
- Grant of a compulsory license on Nexavar and India’s working requirements.
- Administrative delays.
Indonesia
- Patentability criteria.
- 2012 compulsory licensing decree.
- Lack of regulatory data protection.
- Lack of patent term extension.
- Counterfeit medicines.
- Annuity payments and back taxes on withdrawn patents.
- Lack of appropriate plant variety protection.
Thailand
- Support of compulsory licensing as part of trade policy.
- Lack of effective regulatory data protection.
- No formal system of patent linkage.
Russia
- No preliminary injunction against infringement for regulatory approval filing by a generic company.
- Lack of effective regulatory data protection.
- Unclear regulatory standards for orphan drugs.
- Desire to use compulsory licenses.
- Discussion of use of parallel importation with former Soviet countries.
- Counterfeit medicines.
- Discriminatory practices in government procurement.
Turkey
- Unclear patentability criteria for secondary use patents.
- Compulsory license provisions.
- Regulatory delay.
- Short regulatory data protection period.
- Forced localization requirements.
- Good Manufacturing Practices requirements.
- Pricing and reimbursement criteria.
- Lack of orphan drug guidelines and legislation.
Watch List
Australia
- Government request for damages against drug companies that block generic drugs through infringement suits.
Egypt
- Strict patentability requirements.
- Lack of patent linkage.
- Lack of regulatory data protection.
Mexico
- Lack of adequate regulatory data protection.
- Long IP enforcement case times.
- Insufficient patent linkage rules.
South Korea
- Pharmacological data requirements for patent applications.
- Poor implementation of patent linkage requirements under U.S.-Korea FTA.
Vietnam
- Discrimination against pharmaceuticals in violation of TRIPS in patent examination guidelines.
- Counterfeit drugs.
PhRMA
The Pharmaceutical Research and Manufacturers of America (PhRMA) asked USTR to place 12 countries on the Priority Watch List and five countries on the Watch List.
Their Priority Watch List suggestions included:
- India
- Indonesia
- Thailand
- Canada
- Russia
- Turkey
- Argentina
- Brazil
- Colombia
- Ecuador
- Peru
- Algeria
while the Watch List submissions were:
- Australia
- Korea
- Vietnam
- Mexico
- Egypt
PhRMA also suggested that USTR monitor China under section 306 of the Trade Act.
Like BIO, PhRMA criticized various countries for their imagined, proposed, or actual use of compulsory licenses, including Colombia, which sought a compulsory license on imatinib. PhRMA stated their concerns with compulsory licensing in the introduction to their comments:
“Some governments, including India and Indonesia, have issued compulsory licenses that allow local companies to make, use, sell or import particular patented medicines without the consent of the patent holder. Other governments, including Chile, Colombia, Peru, Russia, Turkey and Vietnam, have adopted or are currently considering resolutions, laws and regulations that promote or provide broad discretion to issue such licenses. PhRMA believes governments should grant compulsory licenses in accordance with international rules and only in exceptional circumstances and as a last resort. Decisions should be made on public health grounds through fair and transparent processes that involve participation by all stakeholders and consider all the facts and options.”
PhRMA also cited research by Read Beall, Amir Attaran, and Randall Kuhn to argue that compulsory licenses are not effective in lowering prices:
- Reed F. Beall, Randall Kuhn, and Amir Attaran, “Compulsory Licensing Often Did Not Produce Lower Prices for Antiretrovirals Compared to International Procurement,” Health Affairs 34, no. 3 (March 2015): 493-501.
India
The Alliance for Fair Trade with India (AFTI), an industry interest group run by the well-connected lobbyist Brian Pomper of Akin Gump, asked USTR to place India on the Priority Watch List and to conduct an Out-of-Cycle Review on India this year.
AFTI took particular issue with:
- “forced localization” requirements in the solar industry and for “Internet of Things” products,
- India’s lack of adequate regulatory data protection for pharmaceutical test data and trade secret protections,
- lack of clarity in section 3(k) of the Indian Patents Act with regards to computer software,
- the use of a compulsory license on Nexavar and potential future use of compulsory licenses,
- section 3(d) of the Indian Patents Act,
- amendments to India’s Copyright Act in 2012,
- internet and camcording piracy, and
- the illegal copying of books and written publications.
Though they noted positive developments in India’s patent and copyright policy and law, the U.S.-India Business Council (USIBC) echoed many of AFTI’s concerns in their submission, including with regards to profiting through online advertising by copyright pirates, section 3(d) of the Patent Act, and the use of compulsory licenses. On compulsory licenses, they wrote that “Compulsory licenses should only be granted in exceptional circumstances and as a last resort as they do not provide sustainable solutions to longer term challenges.”
In contrast to AFTI and USIBC, the Confederation of Indian Industry defended India’s patent system, noting efforts to reduce the backlog and improve IPR enforcement. The Confederation also defended India’s right to use TRIPS flexibilities, such as compulsory licensing, and to resist the imposition of TRIPS+ policies, citing with approval the United Nations Secretary-General’s High-Level Panel on Access to Medicines Report, as well as an article by Nobel Prize winning economist Joseph Stiglitz and Senator Bernie Sanders.
Computer & Communications Industry Association (CCIA)
The Computer & Communications Industry Association (CCIA) represents a range of technology companies, and this year submitted comments on the relationship between the cross-border trade in goods and services, intellectual property law, and the internet.
CCIA argued that European ancillary copyright laws, known as “snippet taxes,” harmed U.S. trade with Europe. “The effect of these laws is to force U.S. search providers and other online services to pay simply to quote from publicly available news publications,” they wrote. Germany and Spain received special attention as the “most problematic examples of snippet taxes,” although CCIA also focused on France and EU ancillary copyright efforts.
Moreover, CCIA took note of lack of adequate protections of “U.S. firms operating as online intermediaries” in the EU and Australia.
Notably, CCIA did not ask USTR to use its watch list powers to single out any of the countries discussed in their submission. Instead, CCIA asked that USTR “should make clear that the Berne Convention’s quotation exception and the safe harbor provisions required by the Intellectual Property chapter of U.S. Free Trade Agreements are mandatory commitments that the U.S. Government intends to enforce.”
Chamber of Commerce
The Chamber of Commerce’s Global Intellectual Property Center submitted extensive comments bemoaning “[d]eteriorating market access[,] … [i]nappropriate use of compulsory licensing[,] … Global Proliferation of Illicit Streaming Devices (ISDs)[, and] [m]ultilateral mission-creep impacting IP,” such as the recent High-Level Panel report on access to medicines.
While the Chamber did not make recommendations as to which countries should be included on the watch list, it focused the bulk of its written comments in evaluating perceived failures in the following countries:
- Australia
- Brazil
- Canada
- China
- Colombia
- Ecuador
- India
- Indonesia
- Russia
- South Africa
- Venezuela
The Chamber claimed that the declaration of public interest on Glivec by the Colombian government “undermines the legal certainty critical to an effectively functioning IP regime in Colombia.” With regards to India, the Chamber expressed their concern over India’s support for the findings of the HLP on compulsory licensing, and also attacked section 3(d) of the Patent Act, calling it an “additional ‘fourth hurdle’ with regard to inventive step and enhanced efficacy that limits patentability for certain types of pharmaceutical inventions and chemical compounds.”
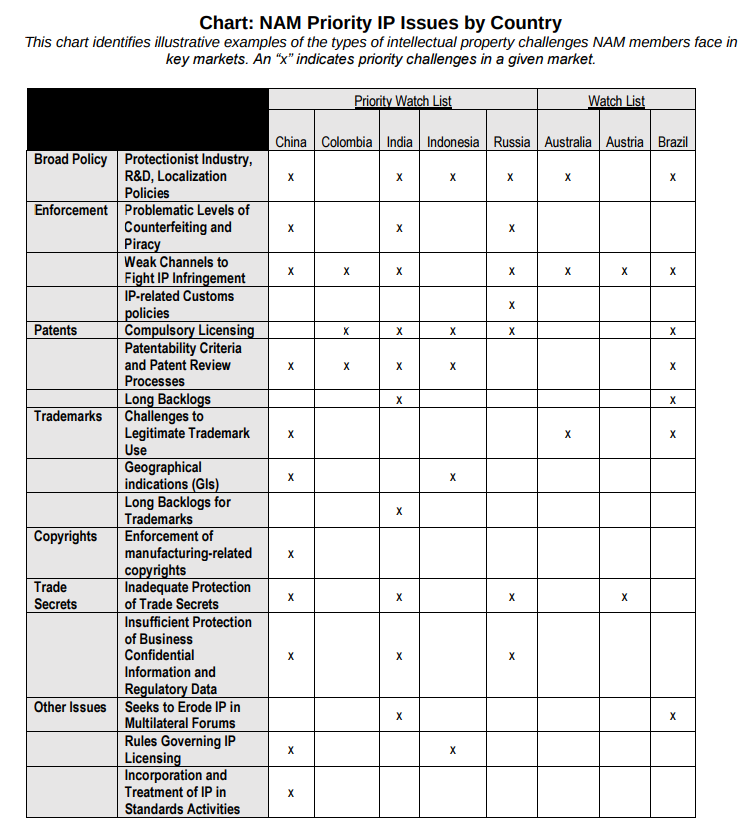
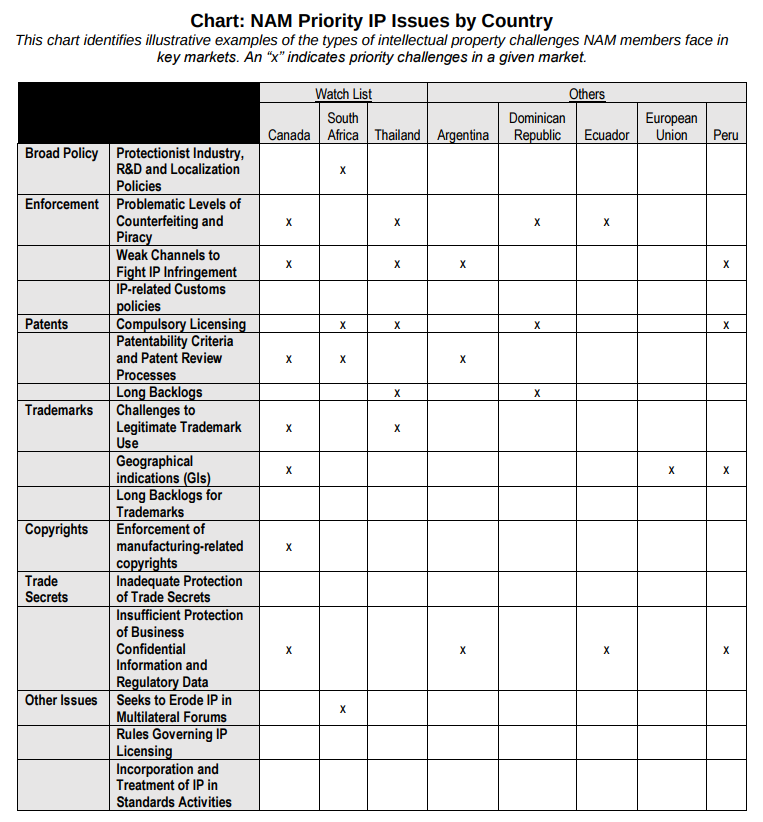
National Association of Manufacturers (NAM)
The National Association of Manufacturers (NAM) provided this helpful chart:
International Intellectual Property Association (IIPA)
The International Intellectual Property Association (IIPA) represents leading U.S. copyright industry groups — including the Association of American Publishers, Entertainment Software Association, Independent Film & Television Alliance, Motion Picture Association of America (MPAA), and Recording Industry Association of America (RIAA) — during the Special 301 process.
For the 2017 list, IIPA recommended the following countries for the Priority Watch List:
- Chile
- China
- India
- Mexico
- Russian Federation
- Taiwan
- Ukraine
- Vietnam
and the following industries for the Watch List:
- Brazil
- Canada
- Colombia
- Indonesia
- Peru
- Switzerland
- Thailand
- United Arab Emirates
IIPA identified “key challenges” to the copyright industry, including:
- internet and mobile network piracy;
- illicit streaming devices;
- TPM circumvention;
- stream-ripping devices;
- illegal camcording;
- book and journal piracy;
- signal theft;
- mobile device piracy;
- pirated disc sales;
- issues with collective management organizations; and
- the need for deterrent enforcement responses.
IIPA also raised concerns about the use of pirated materials in educational contexts, asking USTR to pressure Taiwan to “Take action against book piracy at educational institutions, including against providers of on-demand printouts of pirated e-books, and against digital piracy on online education platforms” and Canada to prioritize a review of its education amendment to the fair dealing exception in the context of “the crisis in the educational publishing market.”
CropLife America
CropLife America, representing various agricultural and pest management interests, focused on the “protection of safety and efficacy data.”