The biologic drug, Humira (adalimumab) is currently the best selling drug in the world, with revenues of more than $16 billion in 2016, and $4.7 billion in the 2nd quarter of 2017 ($52 million per day). 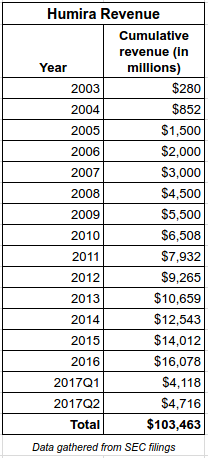
The drug was first approved December 31, 2002 (under Abbott Laboratories from which AbbVie developed as an offshoot in 2013) for the treatment of rheumatoid arthritis. In its first year of sales, Humira earned Abbott $280 million in revenue. Since then, the company has received approval for several new indications. The cumulative revenue from Humira was $103 billion as of June 30, 2017.
In North America and the EU, Humira is now approved to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, plaque psoriasis, hidradenitis suppurativa, juvenile idiopathic arthritis, axial spondyloarthropathy, pediatric Crohn’s disease, and pediatric enthesitis-related arthritis.
This blockbuster of blockbuster drugs has benefited from 5 orphan drug designations (4 approved so far), allowing the company to recoup 50 percent of the costs of trials used to test the drug for the orphan indication.
AbbVie also sought and received patent extensions, which have extended the monopoly in the United States.
Humira is a powerful example of the mismatch between the sizes of the incentives needed for drug development and the incentives companies actually receive.