FDA gives Gilead a seven year regulatory monopoly for remdesivir to treat COVID-19, on grounds it is an “Orphan” treating a rare disease
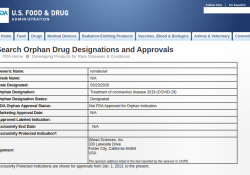
Today the FDA granted Gilead Orphan Drug status for remdesivir for the treatment of COVID-19, on grounds this is a rare disease. The morning of the designation, the U.S. had confirmed, through testing, more than 35 thousand cases, including 8,477… Continue Reading