(Cite as Molly Redfield Ward, Notes on the 2004 to 2009 United States Food and Drug Administration Approval of New Molecular Entities (NMEs), KEI Research Note 2010:3)
A PDF version of this document is available here.
Notes on the 2004 to 2009 United States Food and Drug Administration Approval of New Molecular Entities (NMEs)
KEI Research Note 2010:3
August 5, 2010
Molly Redfield Ward[1]
Knowledge Ecology International
Table of Contents
Introduction
FDA Review Classification
Indications for specific diseases, conditions or treatment function
Distribution of indicated disease in high and low income countries
Results
FDA Review Classification
Categories for Diseases, Conditions and Treatments
WHO Disease Types for high and low income countries
- Table 1: Category for Product Disease or Function
- Table 2: NMEs by Year and FDA Review Classification
- Table 3: New Drug Application Approvals by Year and FDA Review Class
- Table 4: Biologic License Application Approvals by Year and FDA Review Class
- Table 5: Number of NMEs by Category of Disease or Function
- Table 6: FDA Review Classification by Category of Disease or Function
- Table 7: FDA NME approvals by WHO Disease Type, by Year
- Table 8: FDA Review Classification by WHO Type I-III Disease Category
- Figure 1: Distribution of New Molecular Entities
- Figure 2: Priority and Orphan Reviews of New Molecular Entities
- Figure 3: Review Classification by Year
- Figure 4: NMEs by FDA Classification
- Figure 5: Categories of New Molecular Entities
- Figure 6: Percentage of Approvals Receiving Priority Status, by Disease or Function Category
- Figure 7: FDA Review Classification by Category of Disease or Function
- Figure 8: FDA NME approvals by WHO Disease Type, by Year
- Figure 9: FDA Review Class by WHO Disease Type
Introduction
One of the United States Food and Drug Administration (FDA) most important responsibilities is the regulation of prescription medicines, particularly the evaluation of the safety and efficacy of new prescription drugs and vaccines. New prescription drugs or vaccines are regulated under procedures for a New Drug Application (NDA), or a Biologic License Application (BLA).
An NDA consists of the drug’s entire history, from formulation ingredients, to the results of animal and human testings, the biological reaction of the body and the manufacturing, as well as processing and packaging systems. It must provide enough evidence of the pharmaceutical product’s safety and efficacy to receive marketing approval by the FDA.[2] The regulatory approval for an NDA is provided by the FDA’s Center for Drug Evaluation and Research (CDER).
The regulatory review of biological drugs, vaccines or recombinant proteins used in medical treatments is similar in some respects to the process for a pharmaceutical drug. The BLA is the request to the FDA to market a biological compound, providing a detailed history of the fundamental chemistry, medicinal effects, pharmacology as well as the manufacturing process. The regulatory approval of a BLA, however, is provided by the FDA’s Center for Biologics Evaluation and Research (CBER).[3 ]
Each year hundreds of regulatory applications concerning prescription drugs and biological compounds are considered by the FDA, including those seeking approval for new formulations or uses of existing compounds, as well as the approval of completely new compounds, called new molecular entities (NME)s. A NME is defined by the FDA as follows:
…an active ingredient that has never before been marketed in the United States in any form.[4]
This classification is a particularly important category of new medicines. Tracking the number and nature of such products is an important measure of the benefits of public and private sector investments in biomedical research and development, and their impact on health outcomes. The review process of the NME is important to providing additional insight into the nature of the compound relative to existing drugs on the market.
The purpose of this document is to gain a better understanding of the nature of the new molecular entities that were approved between 2004 and 2009. The NMEs were described in terms of the following characteristics:
- FDA Review Classification, including designation under the Orphan Drug Act
- Indications for specific diseases and conditions
- Prevalence of indicated disease in high and low income countries
FDA Review Classification
The FDA defines a Priority Review (P) as:
drugs that offer major advances in treatment, or provide a treatment where no adequate therapy exists. A Priority Review means that the time it takes FDA to review a new drug application is reduced. The goal for completing a Priority Review is six months.[5]
Advances worthy of a Priority Review are demonstrated by:
evidence of increased effectiveness in treatment, prevention, or diagnosis of disease; elimination or substantial reduction of a treatment-limiting drug reaction; documented enhancement of patient willingness or ability to take the drug according to the required schedule and dose; or evidence of safety and effectiveness in a new sub-population, such as children.[6]
The FDA defines a Standard Review (S) as:
a drug that offers at most, only minor improvement over existing marketed therapies[7]
Orphan classification (O) is defined as an entity that treats less than 200,000 Americans, as defined under the Orphan Drug Act of 1983.[8] The orphan designation does not indicate what FDA review classification the NME will receive.
This data was collected from FDA’s New Molecular Entity Drug and New Biologic Approvals report.[9]
Indications for specific diseases, conditions or treatment function
All of the NMEs were further classified into 27 different categories based on the function or structure of the compound, listed here:
Table 1: Category for Product Disease or Function
| 1. Addiction | 15. Growth Hormone |
| 2. AIDS | 16. Heart Disease |
| 3. Anti-fungal | 17. Hepatitis B |
| 4. Antibiotic | 18. Immunosuppressants |
| 5. Antihemorrhagics | 19. Kidney |
| 6. Arthritis | 20. Malaria |
| 7. Cancer | 21. Miscellaneous |
| 8. Diabetes | 22. Over Active Bladder |
| 9. Diagnostic Aid | 23. Pain |
| 10. Dispersing Enhancement | 24. Parkinson’s Disease |
| 11. Diuretics | 25. Psychological |
| 12. Eye | 26. Respiratory |
| 13. Gastrointestinal | 27. Skin Condition |
| 14. Genetic Condition |
Drugs for specific health problems were focused on initially, such as drugs for AIDS, cancer, diabetes, heart disease, kidney disease, malaria, Parkinson’s disease, and psychological conditions, as well as new antibiotics. The remaining classifications emerged from the drugs that did not fall into the above categories. A dispersing enhancement is a drug that aids in the absorption and distribution of another agent. Diagnostic aids are used with imaging machines to improve the visualization of lesions, tumors or other abnormal formations on internal structures. Three drugs were classified twice, as they treated conditions for both heart disease and kidney disease. The duplicates in classification are noted in the title of the graphs (N=145 or N=148).
Distribution of indicated disease in high and low income countries
The NMEs were also assigned a value based on the prevalence of the disease in lower income countries, using the WHO’s Type I, Type II and Type III classification, quoting here from the WHO CIPIH:
Type I disease. Diseases that are incident in both rich and poor countries, with large numbers of vulnerable population in each. Examples of communicable diseases include measles, hepatitis B, and Haemophilus influenzae type b (Hib), and examples of noncommunicable diseases abound (e.g. diabetes, cardiovascular diseases, and tobacco-related illnesses).
Type II disease. Diseases that are incident in both rich and poor countries, but with a majority of cases in poor countries. Type II diseases are often termed neglected diseases.
Type III disease. Diseases that are those that are overwhelmingly or exclusively incident in the developing countries, such as African sleeping sickness (trypanosomiasis) and African river blindness (onchocerciasis). Type III diseases are often termed “very neglected diseases.”[10]
Results
For the six year period of 2004 to 2009, there were a total of 145 NMEs approved by the FDA, of which 123 (86%) were pharmaceuticals and 22 (14%) were biologics.
The annual average of NMEs was 24.2, including 11.8 (49%) that received priority review status, and 7.3 (30%) that received designation as orphan products.
The percentage of orphan designations remained fairly stable throughout the years, ranging from 23% to 35%.
The percentage of priority reviews was high earlier in this time period, peaking at 75% in 2005 and dropping to 32% in 2009.
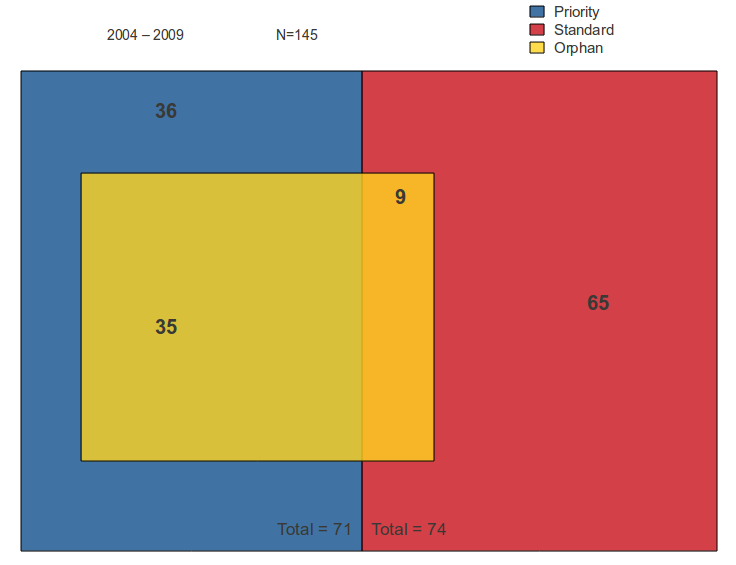
80% of the orphan reviews received priority reviews. Of the priority reviews, approximately 49% were for orphan drugs. Of the standard reviews, only 12% were for orphan drugs.
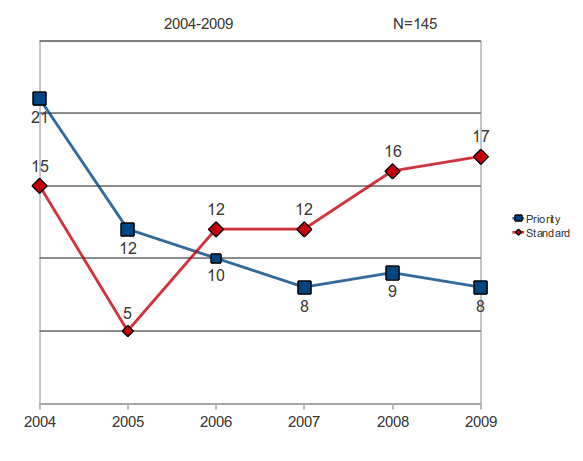
There is a downward trend in the number of priority approvals, and an upward trend for products receiving a standard review status applications continue to rise. The shift from priority to standard review status is an indication of a decrease in productivity.
FDA Review Classification
Table 2: NMEs by Year and FDA Review Classification
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | Total | Average | |
| Standard Review | 15 | 5 | 12 | 10 | 15 | 17 | 74 | 12.3 |
| Priority Review | 21 | 15 | 10 | 8 | 9 | 8 | 71 | 11.8 |
| Total NMEs | 36 | 20 | 22 | 18 | 24 | 25 | 145 | 24.2 |
| Orphan Designation | 10 | 7 | 5 | 6 | 8 | 8 | 44 | 7.3 |
| % priority | 58% | 75% | 45% | 44% | 38% | 32% | 49% | |
| % orphan | 28% | 35% | 23% | 33% | 33% | 32% | 30% | |
| % NDAs | 86% | 90% | 82% | 89% | 88% | 76% | 85% | |
| % BLAs | 14% | 10% | 18% | 11% | 13% | 24% | 15% | |
Figure 1: Distribution of New Molecular Entities
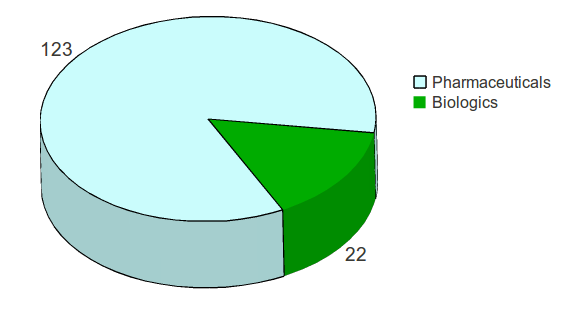
Figure 2: Priority and Orphan Reviews of New Molecular Entities
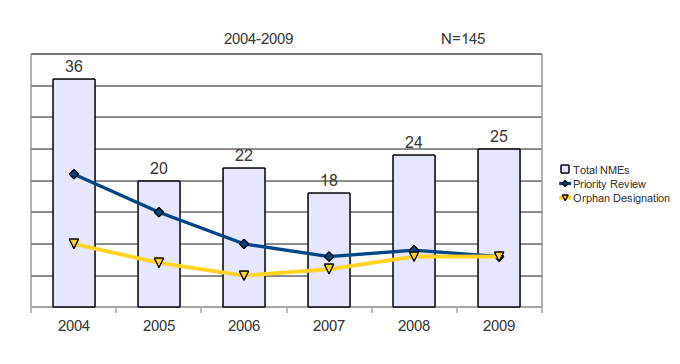
Figure 3: Review Classification by Year
Of the pharmaceuticals drug applications approved, 45% received priority reviews and 28% received orphan designation.
The highest percentage of priority reviews came in 2005 with 72% and the lowest came in 2009 with 26%, showing a downward trend similar to all priority reviews.
The percentages of drugs with orphan designations remained fairly stable, ranging from 17% to 33% throughout this period.
An average of 20.5 drugs were approved per year, of which 9.2 were priority review and 5.7 received orphan designation.
Table 3: New Drug Application Approvals by Year and FDA Review Class
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | Total | Average | |
| Standard Review | 14 | 5 | 12 | 9 | 14 | 14 | 68 | 11.3 |
| Priority Review | 17 | 13 | 6 | 7 | 7 | 5 | 55 | 9.2 |
| Total NDAs | 31 | 18 | 18 | 16 | 21 | 19 | 123 | 20.5 |
| Orphan Designation | 10 | 6 | 3 | 5 | 6 | 4 | 34 | 5.7 |
| % priority | 55% | 72% | 33% | 44% | 33% | 26% | 45% | |
| % orphan | 32% | 33% | 17% | 31% | 29% | 21% | 28% | |
Of the biological license applications, 73% received priority review and 45% received orphan designation.
The percentages of biologics that received priority reviews was high, consistently above 50% and reaching 100% in 2005 and 2006.
The percentage of orphan designations each year was additionally strong at either 50% or 67% for four of the six years. There was only one year with no orphan designations.
An average of 3.7 biologics were approved each year, of which 2.7 were priority reviewed and 1.7 were orphan designations.
Table 4: Biologic License Application Approvals by Year and FDA Review Class
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | Total | Average | |
| Standard Review | 1 | 0 | 0 | 1 | 1 | 3 | 6 | 1.0 |
| Priority Review | 4 | 2 | 4 | 1 | 2 | 3 | 16 | 2.7 |
| Total BLAs | 5 | 2 | 4 | 2 | 3 | 6 | 22 | 3.7 |
| Orphan Designation | 0 | 1 | 2 | 1 | 2 | 4 | 10 | 1.7 |
| % priority | 80% | 100% | 100% | 50% | 67% | 50% | 73% | |
| % orphan | 0% | 50% | 50% | 50% | 67% | 67% | 45% | |
Figure 4: NMEs by FDA Classification
Categories for Diseases, Conditions and Treatments
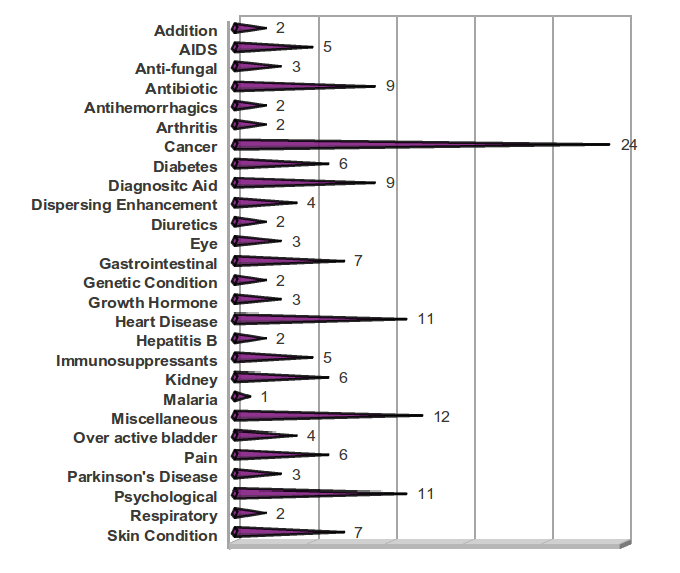
The greatest number of drugs approved under these classifications were for cancer, with 24 drugs approved during these six years. Drugs for psychological conditions and heart disease were also quite prominent with 11 drugs approved each. Drugs for neglected tropical diseases were disappointingly although not entirely unexpectedly low, as only one drug was approved during this time period – a product for malaria that had already been on the international market for several years.[11] The number of AIDS drugs was significant, with almost one new drug approved each year. The high number of new antibiotics approved is also notable, as it is an area of major concern in recent years due to the increasing resistance to existing antibiotics.
Table 5: Number of NMEs by Category of Disease or Function
| Disease or Function | Number of NMEs |
| Malaria | 1 |
| Skin condition | 2 |
| Respiratory | 2 |
| Diuretics | 2 |
| Antihemorrhagics | 2 |
| Hepatitis B | 2 |
| Genetic condition | 2 |
| Arthritis | 2 |
| Addiction | 2 |
| Parkinson’s disease | 3 |
| Growth hormone | 3 |
| Eye | 3 |
| Anti-fungal | 3 |
| Over active bladder | 4 |
| Dispersing enhancement | 4 |
| Immunosuppressants | 5 |
| AIDS | 5 |
| Pain | 6 |
| Kidney | 6 |
| Diabetes | 6 |
| Gastrointestinal | 7 |
| Diagnostic Aid | 9 |
| Antibiotic | 9 |
| Psychological | 11 |
| Heart disease | 11 |
| Miscellaneous | 12 |
| Cancer | 24 |
Figure 5: Categories of New Molecular Entities
There are important differences between the treatment categories with regard to FDA review classification. In looking at the percentage of approvals that received priority status:
All 21 products approved for diabetes, diuretics, psychological or respiratory illnesses received a standard review.
All 21 products approved for addiction, AIDS, antihemorrhagics, dispersing enhancements, genetic conditions, immunosuppressants and malaria received a priority review.
Despite the often discussed need for new antibiotics, only 11% of the NMEs that were classified as antibiotics received a priority review classification, meaning the majority of new antibiotics provide only marginal benefits over existing regimens.
Ten treatment categories had 33 percent or lower priority reviews.
Twelve treatment categories had 67 percent or higher priority reviews.
Table 6: FDA Review Classification by Category of Disease or Function
| Categories | Priority | Standard | Percentage of Priority Review | Orphan |
| Diabetes | 0 | 6 | 0% | 0 |
| Diuretics | 0 | 2 | 0% | 0 |
| Over active bladder | 0 | 4 | 0% | 0 |
| Psychological | 0 | 11 | 0% | 2 |
| Respiratory | 0 | 2 | 0% | 0 |
| Antibiotic | 1 | 8 | 11% | 1 |
| Kidney | 1 | 5 | 17% | 1 |
| Gastrointestinal | 2 | 5 | 29% | 3 |
| Diagnostic Aid | 3 | 6 | 33% | 1 |
| Parkinson’s disease | 1 | 2 | 33% | 1 |
| Miscellaneous | 5 | 7 | 42% | 5 |
| Heart disease | 5 | 6 | 45% | 3 |
| Arthritis | 1 | 1 | 50% | 0 |
| Hepatitis B | 1 | 1 | 50% | 0 |
| Skin condition | 1 | 1 | 50% | 1 |
| Anti-fungal | 2 | 1 | 67% | 0 |
| Eye | 2 | 1 | 67% | 0 |
| Growth hormone | 2 | 1 | 67% | 3 |
| Pain | 4 | 2 | 67% | 0 |
| Cancer | 19 | 5 | 79% | 14 |
| AIDS | 5 | 0 | 100% | 0 |
| Addiction | 2 | 0 | 100% | 0 |
| Antihemorrhagics | 2 | 0 | 100% | 2 |
| Dispersing enhancement | 4 | 0 | 100% | 0 |
| Genetic condition | 2 | 0 | 100% | 2 |
| Immunosuppressants | 5 | 0 | 100% | 4 |
| Malaria | 1 | 0 | 100% | 1 |
Figure 6: Percentage of Approvals Receiving Priority Status, by Disease or Function Category
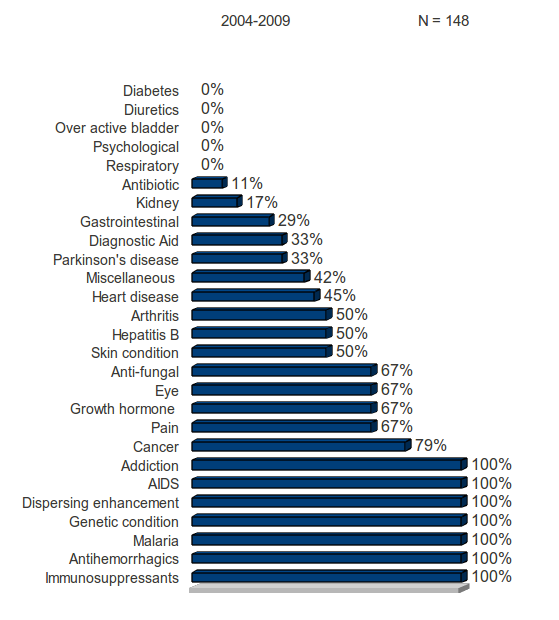
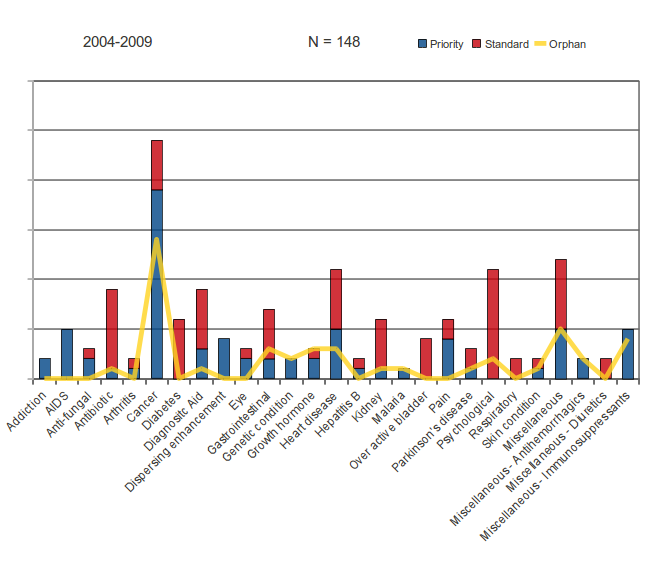
Figure 7: FDA Review Classification by Category of Disease or Function
WHO Disease Types for high and low income countries
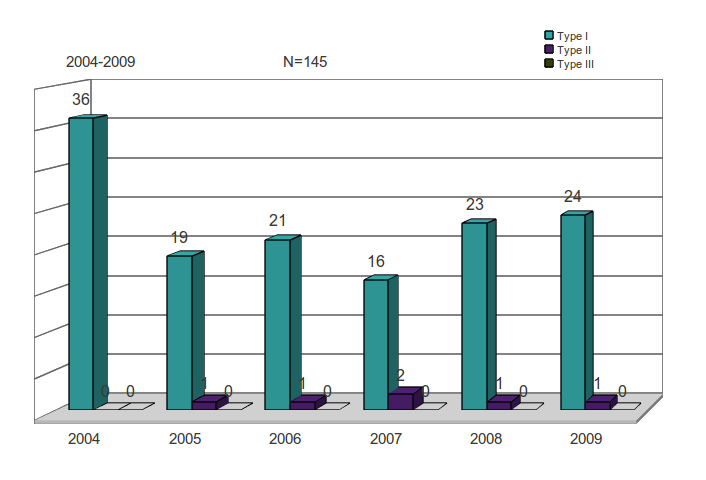
Of the 145 NMEs approved by the FDA between 2004 and 2009, 139 were for Type I diseases – more than 90% for approvals in every year.
Just six Type II disease products were approved over all, one each year in 2005, 2006, 2008 and 2009, none in 2004 and two in 2007.
There were no Type III drugs approved.
Table 7: FDA NME approvals by WHO Disease Type, by Year
| Year | Type I | Type II | Type III |
| 2004 | 36 | 0 | 0 |
| 2005 | 19 | 1 | 0 |
| 2006 | 21 | 1 | 0 |
| 2007 | 16 | 2 | 0 |
| 2008 | 23 | 1 | 0 |
| 2009 | 24 | 1 | 0 |
Figure 8: FDA NME approvals by WHO Disease, Type by Year
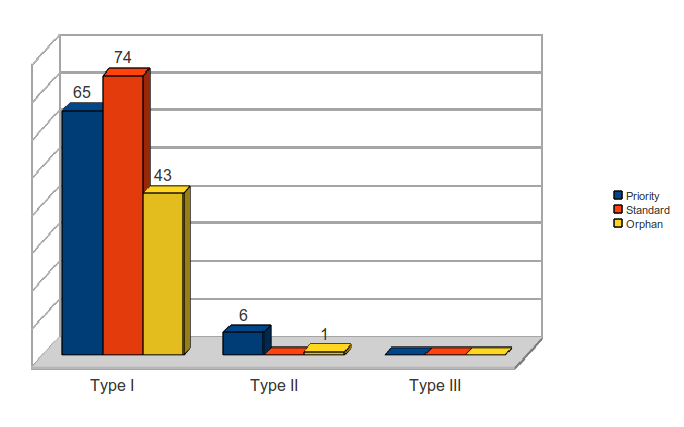
Of the Type I diseases, 65 were priority reviews and 74 were standard review. There were a total of 43 orphan drugs for Type I diseases.
Of the Type II diseases, all 6 were priority review. Only 1 was an orphan drug.
There were no Type III diseases.
Table 8: FDA Review Classification by WHO Type I-III Disease Category
| Disease Type | Priority | Standard | Orphan |
| Type I | 65 | 74 | 43 |
| Type II | 6 | 0 | 1 |
| Type III | 0 | 0 | 0 |
Figure 9: FDA Review Class by WHO Disease Type
1 The Author thanks Ruth Lopert, Judit Rius, Malini Aisola, Anne Mira Guha, Alberto Cerda, Dana Leary and James Love for helpful comments on an earlier draft.
2 Drugs@FDA Glossary of Terms
3 Ibid
4 Drugs@FDA Glossary of Terms
5 Ibid
6 Ibid
7 Ibid
8 Ibid
9 http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/ucm121136.htm
10 Public health, innovation and intellectual property rights: report of the Commission on Intellectual Property Rights, Innovation and Public Health. Geneva, World Health Organization, 2006. The definitions are based upon this earlier report: Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health. Geneva, World Health Organization, 2001.
11 The US registration of the malaria drug Coartem (Artemether; Lumefantrine 120) by Novartis was motivated by the new Priority Review Voucher (PRV) program. The PRV rewards the registration of neglected diseasses with a voucher that grants an expeedited regulatorty review to a separate product choosen by the holder of the voucher, that would otherwise have a standard review time.