CR-prize-study-tor-2024 At the end of 2024, Congress was poised to enact a 1547 page appropriations bill with bipartisan support. Elon Musk blew up the bill, mounting an attack on the bill through X, the social media platform he controls. The… Continue Reading →
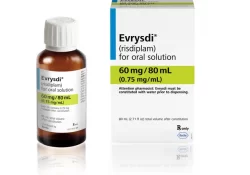
Cost of initial risdiplam trials The cost of trials used to support the initial FDA approval of risdiplam (Evrysdi) KEI Research Note 2025:2 James Packard Love April 23, 2025
On Friday, 22 January 2021, Knowledge Ecology International delivered the following remarks on WHO”s Global Observatory on Health R&D at the 148th session of WHO’s Executive Board. At meetings of WHO governing bodies, non-State actors are limited to one minute… Continue Reading →
General Statement of Knowledge Ecology International – Sixty-First Series of Meetings of the Assemblies of the Member States of WIPO (WIPO Assemblies) Agenda item 5 – General Statements 21 September 2020 In relation to the COVID-19 response, Knowledge Ecology International… Continue Reading →
KEI Research Note: 2020-4: The current and potential role of biomedical research mandates 2020 The current and potential role of biomedical research mandates. Progress in biomedical research depends upon funding. This funding comes from a variety of public sector, non-profit… Continue Reading →
Below is a link to an essay on the use of the phrase “global public goods”. The essay focuses on the three papers Paul Samuelson wrote in the 1950s that are often used, and misused, to define public goods. Samuelson’s… Continue Reading →
KEI is an accredited non-state actor at the World Health Organization (WHO). This is our statement at the 73rd World Health Assembly. https://extranet.who.int/nonstateactorsstatements/meetingoutline/6 Knowledge Ecology International Meeting: Seventy-third World Health Assembly Written statements on COVID-19 pandemic The biggest question today… Continue Reading →
The World Intellectual Property Organization (WIPO) is convening an extraordinary session of the WIPO General Assembly from May 7, 2020 to May 8, 2020; the purpose of this extraordinary session of the General Assembly is to appoint Mr. Daren Tang… Continue Reading →
KEI filed these comments on the NIH Draft Policy for Data Management and Sharing and Supplemental DRAFT Guidance January 10, 2019 1621 Connecticut Avenue NW Suite 500 Washington, DC 20009 www.keionline.org January 10, 2020 Office of Science Policy National Institutes… Continue Reading →
On Friday, 18 October 2019, South Africa delivered the following statement at the WTO TRIPS Council on transparency of R&D Costs and Pricing of Medicines and Health Technology. Intellectual Property and the Public Interest: R&D Costs and Pricing of Medicines… Continue Reading →