On 20 May 2025, the World Health Assembly (WHA) adopted the WHO Pandemic Agreement “pursuant to Article 19 of the Constitution of the World Health Organization” through resolution WHA78.1. While achieving consensus on a number of fronts including pandemic prevention and surveillance, research and development, transfer of technology, and sustainable financing, the provisions governing the Pathogen Access and Benefit-Sharing System (PABS) including “definitions of pathogens with pandemic potential and PABS Materials and Sequence Information, modalities, legal nature, terms and conditions, and operational dimensions” remain unfinished business. (Source: Article 12.2, WHO Pandemic Agreement).
WHA resolution 78.1 frames the relationship between the Article 12 PABS Annex and the WHO Pandemic Agreement. In essence, the signing, ratification, or accession to the WHO Pandemic Agreement is predicated upon the adoption of the PABS Annex.
3. NOTES, in accordance with Article 31 of the WHO Pandemic Agreement, that the WHO Pandemic Agreement shall be open for signature after adoption of the Annex described in Article 12 of the Agreement by the Health Assembly, at the World Health Organization headquarters in Geneva, and thereafter at United Nations Headquarters in New York, on dates to be determined by the World Health Assembly;
The Pandemic Agreement’s chapeau on pathogen access and benefit sharing (Article 12.1) sets out the principles guiding the forthcoming PABS negotiations.
1. Recognizing the sovereign right of States over their biological resources and the importance of collective action to mitigate public health risks, and underscoring the importance of promoting the rapid and timely sharing of “materials and sequence information on pathogens with pandemic potential” (hereinafter “PABS Materials and Sequence Information”) and, on an equal footing, the rapid, timely, fair and equitable sharing of benefits arising from the sharing and/or utilization of PABS Materials and Sequence Information for public health purposes, the Parties hereby establish a multilateral system for safe, transparent, and accountable access and benefit-sharing for PABS Materials and Sequence Information, the “WHO Pathogen Access and Benefit-Sharing System” (hereinafter the “PABS System”), to be developed pursuant to paragraph 2 of this Article.
In terms of nailing down the nuts and bolts of establishing a PABS system, Article 12.2 of the Pandemic Agreement is not short on ambition:
2. The provisions governing the PABS System, including definitions of pathogens with pandemic potential and PABS Materials and Sequence Information, modalities, legal nature, terms and conditions, and operational dimensions, shall be developed and agreed in an instrument in accordance with Chapter III (hereinafter the “PABS Instrument”) as an annex. The PABS Instrument shall also define the terms for the administration and coordination of the PABS System by the World Health Organization. For the purposes of the coordination and operation of the PABS System, the World Health Organization shall collaborate with relevant international organizations and relevant stakeholders. All elements of the PABS System shall come into operation simultaneously in accordance with the terms of the PABS Instrument.
The Intergovernmental Working Group (IGWG) for PABS discussions are co-chaired by Ambassador Tovar da Silva Nunes of Brazil and Mr Matthew Harpur of the United Kingdom of Great Britain and Northern Ireland.
The timeline for the IGWG meetings are set out here.
- 15–19 September 2025 Second meeting of the IGWG
- 6–10 October 2025 Informal meetings of the IGWG
- 3–7 November 2025 Third meeting of the IGWG
- 1–5 December 2025 Fourth meeting of the IGWG
- 9–14 February 2026 Fifth meeting of the IGWG
- 23–28 March 2026 Sixth meeting of the IGWG
- 18–23 May 2026 Seventy-ninth World Health Assembly – Consideration of the outcome of the IGWG regarding the Annex to the WHO Pandemic Agreement described in Article 12 of the WHO Pandemic Agreement
- July 2026 (tbc) Seventh meeting of the IGWG
- September 2026 (tbc) Eighth meeting of the IGWG to be held potentially back-to-back with the meeting of the States Parties Committee for the Implementation of the International Health Regulations (2005)
In addition to negotiations on the PABS Annex, the Bureau envisions that the 2026 sessions of IGWG will address a “proposal for the terms of reference for the Coordinating Financial Mechanism and the modalities for its operationalization and governance in relation to the implementation of the WHO Pandemic Agreement as described in Article 18 of the WHO Pandemic Agreement, for consideration by the Conference of the Parties.”
For the July 2026 session, the Bureau has proposed the following deliverables:
In preparation for upcoming WHO discussions on pathogen access and benefit sharing, the WHO published submissions from WHO member states to shape the negotiations.
Africa Group
Australia, the United Kingdom, Norway, Canada, and New Zealand
Bangladesh
Brazil
Central African Republic
China (English version)
Colombia
European Union
Group for Equity
Indonesia
Japan
Malaysia
Russian Federation (English version)
South Africa
Switzerland
Tunisia
Türkiye
Australia, the United Kingdom, Norway, Canada, and New Zealand
The joint proposal by Australia, the United Kingdom, Norway, Canada, and New Zealand outlines the type of expertise that the proponents view as requiste to “develop the PABS Annex” reflecting an all hands on deck approach.
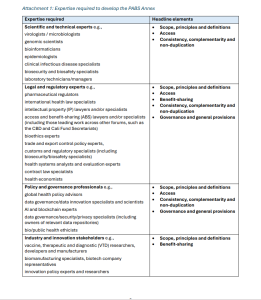
Australia, the UK, Norway, Canada, and New Zealand hold that industry experts including vaccine, therapeutic and diagnostic (VTD) researchers, developers and manufacturers, biomanufacturing specialists, biotech company representatives, innovation policy experts and researchers can contribute to headline elements including: 1) scope, principles and definitions and 2) benefit sharing.
For expertise feeding into discussions on 1) scope, principles and definition, 2) access, and 3) consistency, complementarity and non-duplication the proponents identify scientific and technical experts as relevant experts. These set of experts include:
The joint proposal proponents identify legal and regulatory experts as providing relevant expertise on
• Scope, principles and definitions
• Access
• Benefit-sharing
• Consistency, complementarity and non-duplication
• Governance and general provisions
These legal and regulatory experts would include:
For policy and governance professionals, the joint proposal recommends these experts address:
The policy and governance processionals would include:
In relation to benefit sharing including the “granting of non-exclusive licences to manufacturers in developing countries, for effective production and delivery of [VTDs]; and other forms of transfer of technology as mutually agreed [refer footnote 8 in the WHO Pandemic Agreement], including transfer of relevant knowledge, skills and technical expertise”, the proponents propose the consideration of:
China
In China’s submission, Health Policy Watch gleaned that the “access pharmaceutical manufacturers get to information about dangerous pathogens should be “contingent” on their home country being a party to the Pandemic Agreement”. (Source: China Ties Manufacturers’ Access to Pathogen Information to Host Country’s Commitment to Pandemic Agreement)
Switzerland
Underpinning the Swiss submission is their quest to “visualize how a future PABS System could be operationalized, based on the text (Art. 12) adopted by the WHA on 20 May 2025.”
Switzerland proposed a tabletop simulation to wargame possible scenarios on how a PABS Annex would respond to a pathogen with pandemic potential.
We propose a tabletop simulation, similar to a role play, based on the available draft of the PABS Annex and on the adopted text of the Pandemic Agreement. The Exercise will simulate real-time decisions and processes that States Parties, WHO, Reference Laboratories, participating manufacturers and stakeholders would face when responding to an emerging disease threat related to a pathogen with pandemic potential.
The objectives of the exercise include:
Group for Equity
In their submission for the design of the PABS System, the Group for Equity propose that:
WHO shall be responsible for facilitating the funds required to cover the costs related to shipment of PABS Materials to and within the WHO Coordinated Laboratory Network, by developing country Parties.
In relation to access and benefit sharing, the Group for Equity propose that all recipients accessing PABS materials and/or PABS sequence information “annually contribute x% of total annual revenue for each product or service developed and commercialised using the PABS System”. At this stage, the annual contribution of beneficiaries of the PABS System has yet to be determined.
In terms of licensing, the Group for Equity paper details the following pathway:
in the event of a PHEIC and/or a pandemic emergency, to grant WHO non-exclusive licenses that can be sublicensed to manufacturers in developing countries, especially to those that provided PABS Materials and PABS Sequence Information, for the development and/or production of pandemic-related products needed in developing countries, to expand supply rapidly, ensuring prompt equitable access in developing countries. Such a license to include provision of the full regulatory dossier, technical know-how, and any necessary materials (e.g.cell-lines etc.).