At the February 24, 2015 USTR hearing on Special 301, KEI asked to provide supplemental comments on R&D for the record, and KEI was separately asked by USTR to provide comments on online pharmacies, and by DHHS to comment on the relationship between emergencies and compulsory licensing. (The KEI page on Special 301 is here: /ustr/special301).
Below are the answers to the USTR question about online pharmacies, parallel trade and counterfeit drugs.
KEI Response to Question Regarding Online Pharmacies, Parallel Trade and Counterfeit Drugs
In the matter of the Office of United States Trade Representative (USTR) Notice: 2015 Special 301 Review: Identification of Countries Under Section 182 of the Trade Act of 1974.
Docket ID: USTR-2014-0025
February 27, 2015
At the February 24, 2015 hearing, KEI was asked to comment on the issue of online pharmacies, parallel trade, and counterfeit drugs, in the context of comments submitted to the record by Attaran and Beal regarding Canadian online pharmacies.
KEI is of course opposed to the sale of counterfeit drugs, and supports criminal and civil sanctions for such activity, which puts consumers at risk and also undermines the legitimate interests of manufacturers of legitimate products.
KEI has nuanced views regarding parallel trade.
In general, KEI supports legitimate parallel trade among countries of roughly equal incomes.
KEI supports the personal exemption that allows individuals to buy drugs anywhere for importation to the United States for personal use, for a family member, or for similar situations, including situations that occur when consumers are lacking insurance.
KEI supports parallel imports from higher-income countries to lower-income countries, however, generally supports restrictions on commercial parallel trade from low-income to higher-income countries.
KEI believes legitimate online pharmaceuticals offer considerable benefits to consumers, but supports the regulation of online pharmacy sales.
Europe has extensive experience with parallel trade, and this includes the regulation of parallel traders, in order to protect consumers.
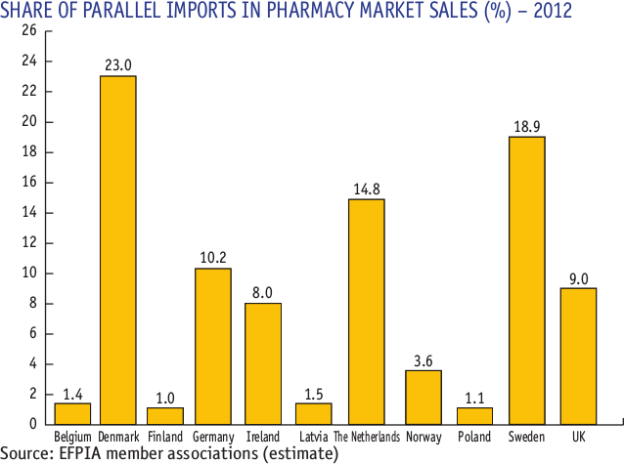
The failure of the United States to have a successful regulatory regime for online parallel trade is for the most part due to PhRMA member companies lobbying against anything that would legitimize parallel trade, even among higher-income countries.
In the past, KEI has asked USTR and customs officials for data on the seizures of counterfeit drugs, and, as far as we know, this information is not organized or accessible. In 2012 we reviewed press releases from the U.S. Immigration and Customs Enforcement (ICE) regarding arrests and prosecutions for the sale of counterfeit drugs in the United States. A summary of our 2012 report follows:
/node/1427Recent ICE Press regarding counterfeit of pharmaceutical drugs
June 1, 2012This is a rough list of the recent ICE press releases mentioning counterfeit and pharmaceutical. It is quite clear that the overwhelming majority of counterfeit busts involve Viagra and other erectile dysfunction drugs, a problem that will probably resolve itself once the Pfizer patents on Viagra expire.
In my quick read of the ICE press releases, I found just 12 pharmaceutical counterfeiting cases in the ICE press releases from 2009 to May 2012, with 14 defendants.
- 13 of the 14 defendants were men.
- 7 defendants appeared to be US citizens. 7 had foreign or mixed nationalities.
- Of the 12 cases, 9 involved erectile dysfunction drugs such as Viagra, Cialis or Levitra.
- The one women defendant was distributing counterfeit Botox.
- One case involved a weight loss product known as “alli”
- In only one case were the counterfeits non-lifestyle drugs (involving Plavix, Zyprexa, Casodex, Tamiflu, and Acricept).
After the February 24, 2015 hearing, KEI Perls Research and Policy Fellow Elizabeth Rajasingh reviewed ICE press releases for 2013 and 2014, and found nine press releases, all of which mentioned Viagra, the number one best-selling erectile disfunction drug, and several also mentioning Cialis, the second highest-selling erectile disfunction drug. There were also mentions of Proscar, a drug for hair loss, and oxycodone, a narcotic.
Given the small number of cases, it seems doubtful that the ICE press releases provide a complete picture of enforcement activity for drug counterfeiting in the United States, but they provide some useful data points.
It would be useful if USTR or other US federal agencies could provide better statistics on the drug counterfeiting law enforcement actions.
However, consistent with our own preliminary review of press releases was a report by Denise C. Barrett that also illustrated a small number of cases mostly involving erectile disfunction and other lifestyle drugs. In a March 13, 2013 memo titled “On Behalf of the Federal Public and Community Defenders Before the United States Sentencing Commission Public Hearing on Counterfeit and Adulterated Drugs,” Barrett notes:
Our review of counterfeit drug prosecutions shows that a sizable number involve counterfeit erectile dysfunction drugs, including counterfeit Viagra®, Cialis®, and Levitra®. Other counterfeit drugs, such as weight loss drugs, anti-anxiety medications, and antidepressants, are prosecuted much less frequently. Here are some examples of cases where the court imposed significant terms of imprisonment under the currently applicable guidelines:In Texas, a defendant convicted of conspiracy to traffic in counterfeit drugs, misbranding, and counterfeiting of trademarks was sentenced to 78 months imprisonment and ordered to pay $1,286,060 in restitution to Eli Lilly Corporation and Pfizer Pharmaceuticals.6
In a Houston case prosecuted under both 18 U.S.C. § 2320 and 21 U.S.C. § 331, the 32- year-old owner of a small business received a sentence of 33 months imprisonment following his conviction for conspiring with others in the People’s Republic of China to traffic in counterfeit goods and trafficking in counterfeit and misbranded pharmaceuticals. The case arose out of the discovery at a mail facility in California of two packages containing about 6,500 loose counterfeit Viagra® pills.7
In the Western District of North Carolina, a 56-year-old man was recently sentenced to 24 months imprisonment for selling counterfeit Viagra® and Cialis® at a convenience store in Charlotte, North Carolina. The court also ordered him to pay a $10,000 fine. The pills had some of the active ingredients of the drugs, but the strength was unknown. He was convicted of conspiracy to violate § 2320(a) and § 331(i) as well as several substantive counts of 18 U.S.C. § 2320(a) and 21 U.S.C. § 331(i).8
In Los Angeles, a 36-year-old “drop shipper” who packaged and shipped more than 160,000 counterfeit drugs, including Viagra®, Cialis®, Valium®, Xanax®, and Lipitor® for a Chinese national living in New Zealand received a sentence of 24 months imprisonment following his conviction for conspiracy to traffic in counterfeit goods, in violation of 18 U.S.C. § 2320. He was also ordered to pay $324,530 in restitution to the pharmaceutical companies that manufactured the brand name products.9
In Colorado, the government recommended, and the court imposed, a top-of-the guideline range sentence of 87 months on the defendant who was convicted of trafficking
in a counterfeit version of the weight loss drug, Alli®. He was also ordered to pay $507,567.94 in restitution, including $417,396.39 to Eli Lilly.10In other cases, defendants have received shorter below guidelines sentences for similar felony offenses,11 or the government has allowed them to plead to a misdemeanor counterfeit offense under 21 U.S.C. §§ 331, 333.12
Footnotes:
6 United States v. Kevin Xu, 4:07-cr-00362-1 (S.D. Tex. 2009).
7 United States v. En Wang, 4:10-cr-00087-1 (S.D. Tex. 2011).
8 United States v. Awni Shauaib Zayyad, 3:10-cr-00243-RJC-DCK-1 (W.D.N.C. 2013).
9 United States v. Francis Ortiz Gonzalez, No. CR-10-136-GW (C.D. Cal. 2013).
10 United States v. Shengyang Zhou, 1:10-cr-00226-PAB-1 (D. Colo. 2011).
11 See, e.g., United States v. Gregory Bochter, No. 6:12-cr-60-orl-18KRS (M.D. Fla. 2012) (8 month sentence of imprisonment imposed on drop shipper for trafficking in about 6000 counterfeit erectile dysfunction drugs from China, in violation of 18 U.S.C. § 2320); United States v. Sarah Knott, No. 8:11- cr-001100-JFM-1 (D. Md. 2012) (2 years probation for trafficking in over 45, 0000 counterfeit Viagra® tablets, in violation of 18 U.S.C. § 2320); United States v. Curtis Henry, No. 6:11-cr-06165-CJS-1 (W.D.N.Y. 2012) (3 years probation for trafficking in 740 counterfeit erectile dysfunction drugs from China); United States v. Frank Fu Jen Huang, 2:04-cr-01298-R-1 (C.D. Cal. 2006) (departure/variance from 78-97 months range of imprisonment to six months of home detention, 2,500 hours of community service, and 5 years probation ).
12 See, e.g., United States v. Ali Jones, 2:08-cr-00887-JWJ-1 (C.D. Cal. 2008) (2 years probation for sale of counterfeit erectile dysfunction drugs advertised on craigslist; government agreed not to charge the defendant with a felony count under 18 U.S.C. § 2320); United States v. Jun Huang, 2:09-cr-01028-CT-1 (C.D. Cal. 2010) (1 year probation for sale of counterfeit drugs in violation of 21 U.S.C. § 331); United States v. David Srulevitch, No. 2:04-cr-01559-R-1 (C.D. Cal. 2005) (5 years probation for making about 700,000 counterfeit Viagra®).
As regards the issue of counterfeit Viagra, we can expect this problem to abate somewhat when generic versions of sildenafil citrate are available in the United States, as they are now in many other countries. To this end, we were surprised to see that Pfizer has received a six-month pediatric extension of the Viagra Orange Book patents, for testing the drug on children.
Delinkage, parallel trade and counterfeit drugs
The practices of parallel trade and counterfeiting drugs are both induced by large differences between prices and manufacturing costs of drugs.
In the delinkage paradigm for funding R&D, prices for drugs are much lower, as commodities produced under competitive conditions, and profit margins are also lower. The reward to innovators is separate from the product price. By ending the distortion between costs and prices, parallel trade or arbitrage within country markets ceases to be problematic from the point of view of funding R&D, and counterfeiting becomes much less attractive.