Update. NIH sent us a 147 word response on November 23, 2018: NIH to KEI regarding Ovensa 23 NOV 2018. The brief reply states that “Prior to posting notices of a proposed grant of exclusive commercialization licenses, the NIH determines . . . that the company applying is qualified both technically and financially” and “We reviewed your comments pertaining to the market, worldwide incidence rates, applicable federal statutes regulations, and pricing and will take them into consideration.” This is similar to responses we have received on other licenses, where the NIH has ignored or rejected every suggestion.
On November 20, 2018, KEI submitted joint comments to the Federal Register notice (83 FR 55556) regarding the “Prospective Grant of Exclusive Patent License: Therapeutics for Insulin Resistance and Non-Alcoholic Fatty Liver Disease/Non-Alcoholic Steatohepatitis (NASH/NAFLD).” This notice was posted in regard to a prospective exclusive license to Ovensa, a firm located in Canada.
The comments submitted by KEI were made on behalf of:
- Organizations
- HealthGAP
- Knowledge Ecology International (KEI)
- Social Security Works (SSW)
- The Young Professionals Chronic Disease Network (YP-CDN)
- Individuals
- Allison Love Mardini (type 2 diabetes patient)
- Brook K Baker
- James Love
The full comments follow below, and a PDF version is available here.
Links to all the comments submitted by KEI in response to previous NIH proposed exclusive licenses are available here.
November 20, 2018
Michael Shmilovich, Esq.
Senior Licensing and Patent Manager
31 Center Drive, Room 4A29, MSC2479
Bethesda, MD 20892-2479
shmilovm@mail.nih.gov
Dear Michael Shmilovich,
We are writing in regard to the notice published in the Federal Register (83 FR 55556), “Prospective Grant of Exclusive Patent License: Therapeutics for Insulin Resistance and Non-Alcoholic Fatty Liver Disease/Non-Alcoholic Steatohepatitis (NASH/NAFLD),” concerning a prospective exclusive license to Ovensa, a firm located in Canada.
The patent rights include all continuing U.S. and foreign patents/patent applications thereof for the following inventions:
- HHS Ref. No. E-103-2013-0, U.S. Provisional Patent Application 61/839,239, “Glucan-Encapsulated siRNA For Treating Type 2 Diabetes Mellitus,” filed June 25, 2013,
- International Patent Application PCT/2014/043924 filed June 24, 2014,
- European Patent Application 14818342.9 filed June 24, 2018, and
- U.S. Patent 10,077,446 filed June 24, 2014 and issued September 18, 2018.
The license includes inventions relating to new methods of treating type 2 diabetes or preventing the progression of insulin resistance to overt diabetes.
According to the notice, the license will be “worldwide,” and the license may or may not, “be limited to products sold that include therapeutic siRNAs encapsulated in nanoparticles made from either glucan based biopolymers and/or Ovensa’s TRIOZANTM (N,N,N-Trimethyl Chitosan) proprietary biopolymer.”
Ovensa appears to be a small company located in Ontario, Canada. The company Linkedin profile lists 4 employees, of which not all appear to be working exclusively for the company. The page for “Management Team” on the Ovensa website lists two persons.
Diabetes is not a rare disease. According to the CDC, more than 30 million Americans have diabetes, and 90% to 95% of them have type 2 diabetes.
According to the IDF Diabetes Atlas Eighth Edition (published in 2017), approximately 425 million adults were living with diabetes; and by 2045, this will rise to 629 million.
Also note that per the IDF Diabetes Atlas, 4 out of 5 persons living with diabetes live in low and middle income countries where access to new medicines is particularly limited due to unaffordable prices. 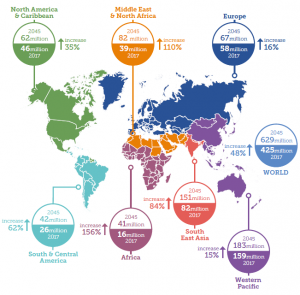
Figure source: IDF Diabetes Atlas Eighth Edition
The NIH has shared a press release published on Marketwatch.com, which provides the following estimates of the market for drugs to treat diabetes.
“The global Antidiabetic Drug market is valued at 49600 million US$ in 2017 and will reach 96700 million US$ by the end of 2025, growing at a CAGR of 10.0% during 2018-2025.”
Cost of development
The NIH has indicated that the most recent $1.38 million in company financing received by Ovensa is evidence that the company has sufficient resources to bring the invention to practical application. The NIH should take into account the relatively modest amount of resources necessary for development when evaluating the appropriate scope of rights for the license, including the term of exclusive rights.
40 USC § 599
At the appropriate time in the licensing process, we expect the NIH to obtain advice from the Attorney General (as is required under 40 USC § 599) to determine if the “disposal to a private interest would tend to create or maintain a situation inconsistent with antitrust law.”
The Bayh-Dole Act provides that “Nothing in this chapter shall be deemed to convey to any person immunity from civil or criminal liability, or to create any defenses to actions, under any antitrust law” [35 USC § 211 – Relationship to antitrust laws].
The Bayh-Dole Act sets out the areas where the Bayh-Dole Act “shall take precedence over any other Act which would require a disposition of rights in subject inventions” [35 USC § 210 – Precedence of chapter], and mentions 21 separate statutes, but does not include 40 USC § 599.
35 USC § 209
Assuming the NIH has conducted a proper analysis to determine if any exclusive rights are necessary to induce investments in research and development (R&D) to bring the inventions to practical application, we ask the NIH to limit the “proposed scope of exclusivity” so that it is “not greater than reasonably necessary to provide the incentive for bringing the invention to practical application,” as is required by 35 USC § 209.
Such an analysis should include an estimate of the expected costs (adjusted for risks and the costs of capital) to bring the invention to practical application, as well as reasonable estimates of the revenue from the sale of the drug or other technology that would be necessary as an adequate incentive for that investment. If the expected investments are small (which seems to be the case given the modest financial resources of Ovensa) and the diabetes market is large, as the NIH suggested with reference to the Wise Guy Reports estimate, then the NIH should limit either (1) the number of years of exclusivity (2) the prices that can be charged, (3) the maximum revenue before exclusivity is reduced or eliminated, or (4) some combination of 1-3.
35 USC § 201(f) – definition of practical application
The Bayh-Dole defines certain terms in 35 USC § 201, including the term “practical application.”
(f) The term “practical application” means to manufacture in the case of a composition or product, to practice in the case of a process or method, or to operate in the case of a machine or system; and, in each case, under such conditions as to establish that the invention is being utilized and that its benefits are to the extent permitted by law or Government regulations available to the public on reasonable terms. [emphasis added]
“Available to the public” and “reasonable terms” taken together include the price to the public being reasonable. For the public, the price is the primary term of the transaction.
Proposals for safeguards to protect the public’s rights in the patented inventions
We propose the following measures to protect the public’s interest in any license to the Canadian firm, Ovensa.
- No discrimination against U.S. residents in pricing
We ask that the NIH include language in the proposed exclusive license to ensure that the prices in the U.S. for any drug, vaccine, medical device or other health technology using the inventions are not higher than the median price charged in the seven countries with the largest gross domestic product (GDP), that also have a per capita income of at least 50 percent of the United States, as measured by the World Bank Atlas Method.
We consider this a modest and indeed minimalist request to protect U.S. residents, who paid for the R&D that created the licensed inventions.
- Additional provisions on affordability
The NIH should require that prices for products in the United States that use the NIH-owned patented inventions do not exceed the estimated value of the treatment, as determined by independent health technology assessments selected by HHS.
The NIH should also create an obligation to set prices low enough that patient co-payments under third party Medicare programs are affordable.
- Reduce term of exclusivity when revenues are large
In addition to an external reference pricing test, we propose that the exclusivity of the license in the U.S. should be reduced when the global cumulative sales from products or services using the inventions exceed certain benchmarks.
Given the modest cost of acquiring an NIH-patented invention, the amount of money the developer needs in sales to justify additional investments in R&D is reduced, as compared to cases where a company develops or acquires the technology from non government sources.
This request is consistent with the statutory requirements of 35 USC § 209, which demands that “the proposed scope of exclusivity is not greater than reasonably necessary to provide the incentive for bringing the invention to practical application.”
One possible implementation of revenue benchmarks is as follows: exclusivity will be reduced by one year for every $500 million in revenue equivalents, earned after the first $1 billion, where revenue equivalent is defined as global cumulative sales plus market entry rewards as well as government grants or tax credits, for the product or products using the invention. However, the NIH could choose different benchmarks, so long as the limits on exclusivity address the requirements of 35 USC § 209, in that the incentive is “not greater than reasonably necessary.”
- Low and Middle Income Countries
In general, we are concerned that several NIH-funded inventions are not accessible in low and middle income countries, due to prices that are high and not affordable in markets where per capita incomes are significantly lower than the United States. For this reason, we generally ask, and are asking in this specific case, that the NIH limit the exclusivity in the license to countries that have per capita incomes that are at least 30 percent of the United States.
We also generally ask the NIH to reach out to the Medicines Patent Pool (MPP), in order to enter into an agreement that gives the MPP an option to negotiate non-exclusive open licenses for the inventions in developing countries.
According to the “United States Public Health Service Technology Transfer Policy Manual, Chapter No. 300, PHS Licensing Policy:”
“PHS seeks to promote commercial development of inventions in a way that provides broad accessibility for developing countries.”
For this license, we ask that the NIH clarify the geographic area of the license, and provide information on the measures that will be taken to ensure the policy of “broad accessibility for developing countries” is actually implemented.
The policy of promoting access seems to have been routinely ignored in the past. The NIH has rejected efforts to restrict the geographic area of exclusive rights or to impose access requirements on companies holding licenses, including in cases involving firms with deplorable records of price gouging worldwide.
We also ask the PHS to reconsider the use of the term “developing countries,” which is no longer the most useful way to describe a category of countries for which access is a challenge.
There is no consensus on how to define “developing countries.” The WTO allows its members to self identify as “developing.”
Policy makers often prefer to use the term “low and middle income countries” (LMIC), but this also requires a thoughtful definition.
The World Bank publishes and updates a list of country classifications every year, but the World Bank definition is anchored in a methodology from the 1980s that was based in part upon the cost of buying food, a poor proxy for global wellbeing today.
The World Bank definition of “high income” was adopted in 1989 by the Bank’s Executive Directors on the basis of a staff report on per capita income measures. The high income threshold was determined by an “explicit benchmark of $6,000 per capita in 1987 prices,” and updated annual with an adjustment for inflation. With real growth in per capita incomes, the number of countries that qualify as high income has continued to rise, and at some point, most countries will probably qualify.
Our recommendation for the NIH is to consider relative per capita income as a useful starting metric for policies designed to mitigate inequality of access, recognizing that in some cases other factors such as prevalence of a disease may be appropriate to consider.
The PCT application referenced in the Federal Register notice identified a very large number of designated countries for foreign patent applications, including the African Regional Intellectual Property Organization (ARIPO), the African Intellectual Property Organization (AIPO), and low income countries in Eastern Europe, Asia, South America and the Caribbean.
| Designated States: | AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW
African Regional Intellectual Property Organization (ARIPO) (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW) Eurasian Patent Office (AM, AZ, BY, KG, KZ, RU, TJ, TM) European Patent Office (EPO) (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, TR) African Intellectual Property Organization (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, KM, ML, MR, NE, SN, TD, TG) |
The WIPO PCT web page for this application notes that after the 30 month period following the priority date, the NIH has only pursued patents at the European Patent Organization and the U.S. PTO, a fact confirmed today by the NIH.
Our concerns about access outside the United States are therefore focused in part on the EPO member states that have per capita incomes significantly lower than the United States.
In 2017, the United States per capita income was estimated by the World Bank to be $58,270. Thirty percent of this figure is $17,481.
We are specifically asking the NIH to exclude from the geographic area of any exclusive right, the following EPO member states, based upon the fact that their 2017 per capita income was less than 30 percent of U.S. per capita income.
Table 1: EPO members, less than 30 percent 2017 U.S. per capita income
- Albania $4,320
- Bulgaria $7,760
- Croatia $12,430
- Hungary $12,870
- Lithuania $15,200
- Latvia $14,740
- Macedonia $4,880
- Poland $12,710
- Romania $9,970
- Serbia $5,180
- Slovenia $16,610
- Turkey $10,930
Test data
In addition, we ask the NIH to include provisions that would require the licensed patent holders to waive any exclusive rights regarding test data and any patent-registration linkage rights that may exist in any country with a per capita income less than 30 percent of U.S. per capita income. This is important because a number of trade agreements and bilateral pressures force low and middle income countries to enact laws granting exclusive rights in test data, in most cases, without the possibility of exceptions, even in cases involving excessive prices.
A provision waiving exclusive rights in test data in countries with lower incomes is necessary for the NIH to implement the PHS policy “to promote commercial development of inventions in a way that provides broad accessibility for developing countries.”
- Transparency
The licensee should be required to file an annual report to the NIH on the research and development costs associated with the development of any product that uses the inventions, including reporting separately and individually the outlays on each clinical trial. We will note that this is not a request to see a company business plan or license application. We are asking that going forward the company be required to report on actual R&D outlays to develop the subject inventions.
Reporting on actual R&D outlays is important for determining if the NIH is meeting the requirements of 35 USC § 209, that “the proposed scope of exclusivity is not greater than reasonably necessary to provide the incentive for bringing the invention to practical application.”
Sincerely,
Organizations
HealthGAP
Knowledge Ecology International (KEI)
Social Security Works (SSW)
The Young Professionals Chronic Disease Network (YP-CDN)
Individuals
Allison Love Mardini (type 2 diabetes patient)
Brook K Baker
James Love