These are the KEI comments on the questions raised by the FDA in the 85 FR 33165 and 85 FR 33169 Federal Register notices.
KEI-comments-FDAOrange Book-31aug2020
KEI comments to the FDA relating to the Approved Drug Products With Therapeutic Equivalence Evaluations (the “Orange Book”)
August 31, 2020
- Introduction
- Federal funding of inventions
- Patent disputes
- Expired patents
- Full term of exclusivity
- Total number of patents for a product, over time
- Clinical trials
- The FDA should also implement changes in the Purple Book
- The FDA should require disclosures about revenue and units sold
- Transparency of product relevant licenses
-
Introduction
-
Federal funding of inventions
-
Patent disputes
-
Expired patents
-
Full term of exclusivity
-
Total number of patents for a product, over time
-
Clinical trials
-
The FDA should also implement changes in the Purple Book
-
The FDA should require disclosures about revenue and units sold
-
Transparency of product relevant licenses
Knowledge Ecology International (KEI) is an advocacy group with offices in Washington, DC, and Geneva, working on issues related to intellectual property and drug pricing. KEI uses the Orange Book frequently for research, for example to determine the patent landscape of approved drug products. We also provide open access to data related to patents, including by sharing the historical Orange Book data files.
KEI appreciates the opportunity to comment on the questions raised in the 85 FR 33165 and 85 FR 33169 Federal Register notices, respectively titled Approved Drug Products With Therapeutic Equivalence Evaluations (the “Orange Book”); Establishment of a Public Docket; Request for Comments and Listing of Patent Information in the Orange Book; Establishment of a Public Docket; Request for Comments.
Our comments focus on data items that we believe should be added to the electronic Orange Book database and the data files.
Over the years, the FDA has constructed the role of the Orange Book narrowly, primarily publishing data items that are strictly necessary to comply with statutory requirements.
This is an opportunity to rethink the scope of information provided to the public, including the needs of academics, policy experts, advocates, health practitioners, businesses and members of the general public who are asking for more transparency and more constructive disclosure of patent landscapes on FDA regulated products, as well as certain information relating to the economic aspects of the development and commercialization of products.
The FDA should require additional disclosures and publish them as data items in the Orange Book. These additional disclosures regarding patents should include:
Patents. Information about patent licenses that companies needed to acquire, disputes over patent validity and infringement, and information that has already been disclosed and is currently available should continue to be reported, including the relevant dates and patent numbers for all patents ever listed for a product, government rights if any in any listed patents, even if the patents have subsequently expired or been removed from the Orange Book.
Clinical Trials used for FDA approvals, including but not limited to trials where applicants claim rights in test data. This information should include the trial identifier, such as the NCT number, the patient enrollment, trial costs and information regarding the funding of the trials including the cost of the trial and the percentage of costs paid the U.S. government, other governments, charities, and industry.
Under 35 U.S.C. § 202(c)(1), any contractor that receives funding from the federal government is required to “disclose each subject invention to the Federal agency within a reasonable time after it becomes known to contractor personnel responsible for the administration of patent matters.”
Under 37 C.F.R. § 401.3(a), federal funding agreements shall contain the “standard patent rights clause” found at 37 C.F.R. § 401.14, barring specific circumstances and exceptions. Subsection (c)(1) of the patent rights clause outlines the disclosure requirements.
Pursuant to 35 U.S.C. § 202(c)(6) and 37 C.F.R. § 1.77(b)(3), contractors are required to state within the patent application or patent that the federal government contributed funding to support the discovery of the invention and that the government retains certain rights. According to 37 C.F.R. § 401.14(f)(4) and NIH Guidelines for Grants and Contracts, grant recipients must include the following language in their patent applications and patents: “This invention was made with Government support under (grant/contract number) awarded by the (Federal agency). The Government has certain rights in the invention.”
In some cases, patent holders have made an assignment of rights to the U.S. government.
Presidential Executive Order 9424, on the Establishment of a Register of Government Interests in Patents, has been implemented by the U.S. Patent and Trademark Office, pursuant to 37 CFR § 3.58 – Governmental Registers, and 37 CFR 1.12: Assignment Records Open To Public Inspection.
The USPTO public database of patent assignments can be challenging to search, and incomplete as to assignments. The separate USPTO Registry of Government Interests in Patents is not open to the public.
The U.S. Patent and Trademark Office does maintain a full text database of patents granted after 1975, which includes a searchable code, GOVT, for declarations in the patent of government interests. However, often patent holders fail to include such disclosures in patent applications, and add them later as certificates of correction to patents. The certifications of corrections are coded in the USPTO database as CofC entries, but the data in the CofC entries are scanned images, not text searchable, and the majority of CofC entries are on other topics, such as names of inventors, patent specifications, etc.
Inventors often fail to comply with disclosure obligations, but when they do disclose federal grants this information provides insights into the role of the U.S. government in the research and development that led to the patented invention, changes the narratives about how R&D was funded, and gives the public and the government information about the rights that are available to the public and the government for both upstream and downstream use of inventions.
KEI requests the FDA to add information about government disclosures on patents listed in the Orange Book. This information can be added in several ways. The ideal approach would be to include this in the list of data items that NDA applicants or NDA holders are required to provide under 21 C.F.R. §§ 314.50(h), 314.53, and 314.70(f)). If an NDA applicant or NDA holder already acknowledged U.S. federal government funding in their patent documents, making these additional disclosures would not significantly increase their paperwork burden. Another approach could be to collaborate with the USPTO, which has a dataset (likely underinclusive, since it tends to exclude cases that initially failed to disclose federal funding but did so later via certificates of corrections) of patents that disclosed U.S. government grants, as well as assignments or rights by patent holders to U.S. agencies
If patent applicants comply with the provisions set forth in 37 C.F.R. § 401.14(f)(4) and explained above, their disclosures will also have the number of the relevant grants or contract numbers. The Orange Book should also include this information in listed patents.
Since the Orange Book is used as a data source to understand the patent landscape of approved drug products, it should also include information about past and ongoing litigation about those rights. KEI asks the FDA to require disclosures about previous and current litigation, if any, for each patent listed in the Orange Book. This should not be limited only to legal actions seeking to delist a patent from the Orange Book. All legal events concerning each patent should be provided, including, for example, disputes over infringement and validity, failure to disclose government rights as required under 35 U.S.C. § 202(c)(6) and 37 C.F.R. § 1.77(b)(3), pending and past march-in requests, 28 U.S.C.§ 1498 cases, or inter partes reviews at the USPTO.
Currently, “[p]atents and exclusivities that have expired are removed from the Orange Book.” Expired patents are removed both from the online database and also excluded from the data files that are available in the FDA website. For reasons further explained below, removing expired patents from the Orange Book limits the type of research insights that can be drawn from studying the listings. We ask the FDA to either retain a list of expired patents in the Orange Book but clearly state that they have expired; or periodically publish a separate list with all of the patents that were ever listed in the Orange Book, including those that have expired. Regardless of how the FDA decides to publish them, this information should be made available in a database that can be searchable by end-users and also in data files with an open format.
It is possible to obtain “previous editions of the Orange Book or an Orange Book Data File” by filing Freedom of Information Act (FOIA) requests, according to a draft guideline recently circulated by the FDA. We know this is in fact the case since on April 27, 2018, KEI requested a copy of the Orange Book data files. About a week later the FDA gave us a data file containing all patents listed in the Orange Book from inception through mid-2018. Since then we have provided open access to this data file on one of our websites and we appreciate the fact that it was provided to us in an open format. We believe, however, that the FDA should publish this information periodically on their website without requiring interested parties to file FOIA requests each time they need updated information. We believe that the need to file FOIA requests discourages some potential researchers or observers in general from exploiting this data source in their analyses.
In addition to releasing the previous editions in response to FOIA requests, the FDA provides a list of patents that have been recently delisted. However, as the FDA explains, this list only includes patents “that have been delisted since the most recent Annual Edition of the Orange Book.” We searched this database on August 4, 2020 and found only 162 entries with just 44 unique patents. This is clearly just a small fraction of all the patents that have ever been delisted from the Orange Book. Although useful, this resource is underinclusive and inadequate for researchers seeking to understand the historical patent landscape of a drug or a group of drugs.
Removing expired or delisted patents from the database and data files has consequences in terms of transparency. Primarily, removing these patents limits the type of historical insights that can be drawn from analyzing Orange Book listings. For instance, the public deserves to know how many approved drugs had patents that disclose U.S. government grants, or were later determined to be invalid or the claims not relevant to the product. This type of analysis would provide insights into the role of the U.S. government in the research and development of new drug products. At least for small molecule drugs, the Orange Book could be a useful tool to answer this question. Researchers and the public in general should be able to use the Orange Book to find drug-patent pairs that disclosed U.S. government grants and to estimate the total number of approved drug products that benefited from public funds, or how frequently this occurs. However, since the Orange Book only lists patents that have not yet expired, using the current version would underestimate the number of drugs linked to federal grants.
Table 1 provides an example that illustrates this problem. The current entry for Janumet, an anti-diabetes drug approved by the FDA, shows four patents listed in the Orange Book. None of these patents discloses grants from the U.S. government and all are assigned to Merck. However, an analysis based on the legacy data files obtained by KEI via FOIA request shows that there were four additional patents listed in connection with Janumet, which have now expired and were therefore removed from the current Orange Book edition. Three of these four patents are assigned to Tufts College, name Daniel Drucker as one of the co-inventors, and disclose U.S. government grants. Therefore, because the Drucker patents have already expired and been removed from the listing, an analysis of Janumet based on the current Orange Book would provide an inaccurate picture of the role of the U.S. government in the research and development that led to this drug.
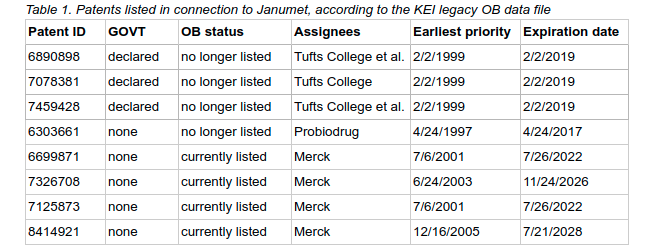
Again, KEI is able to do this kind of analysis because we FOIA’ed and obtained a dataset with all of the patents ever listed in the Orange Book. We used that file, a dataset of publicly-funded inventions available via the USPTO website, and a data analytics tool to find examples of drugs that have expired patents disclosing U.S. government grants. However, not everyone interested in this type of information will be able or willing to FOIA these files to conduct similar analyses.
Most small-molecule drugs list several patents in the Orange Book. Many of them are directed to small inventive contributions that, nevertheless, expand the length of the exclusive protection for several additional years after the expiration of the compound patents. These secondary patents can have huge implications on access and public spending for health technologies. There is a strong public interest in further understanding secondary patents, including how they impact the length of the term of exclusivity. The Orange Book can contribute to this type of research.
KEI asks the FDA to publish Orange Book data files with dates that will allow researchers to estimate the number of years a specific drug has been or will be under some form of exclusivity.
Publishing and regularly updating data files with these dates would encourage further research around secondary patenting and help answer questions relating to, for example, how long a drug is typically under some form of exclusivity or whether the trend has been changing recently.
The FDA should state that the specific product has been under exclusivity for “N years.” This should be searchable by end users.
There are several approaches the FDA can use to do this. For example, the FDA should publish data files with at least four dates for each application or active ingredient: 1) the earliest non-provisional priority date, 2) the drug approval date, 3) the earliest patent expiration date; and 4) the latest patent expiration date. By providing the earliest priority date these data files would reflect the length of exclusivity starting from the moment generally considered to be the closest to the conception of the invention. The inclusion of the drug approval date will reflect the term of protection since a drug was actually launched in the market. With the inclusion of the earliest and the latest patent expiration date researchers may be able to determine how many additional years a specific drug has been or will be under exclusivity due to monopolies over secondary inventions.
To illustrate how adding these dates would improve transparency, KEI used a legacy version of the Orange Book obtained via FOIA request and a data analytics tool to find drugs that have been under patent protection for an abnormally long number of years. Table 2 provides some examples based on our analysis. This analysis is based on the earliest and latest expiration dates of all of the patents listed in the legacy Orange Book for these applications. The examples shown below are just a handful of the cases we found that are in a similar situation.
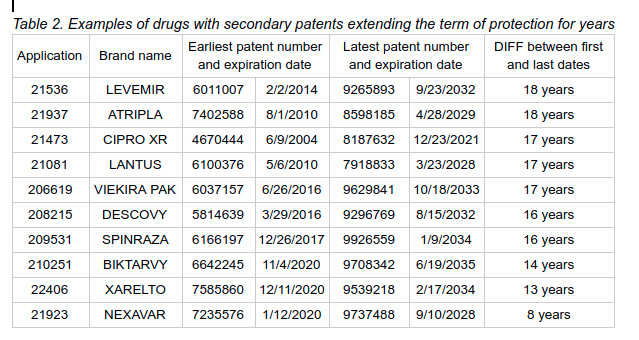
As Table 2 shows, there are drugs listed for which the first patent listed in the Orange Book expired many years before the last patent. Spinraza, for example, originally listed U.S. patent 6,166,197 (the ‘197 patent). The ‘197 patent, which was titled Oligomeric compounds having pyrimidine nucleotide (S) with 2’and 5 substitutions and assigned to ISIS Pharmaceutical, expired on December 26, 2017. Nevertheless, the legacy Orange Book shows that there have been eight additional patents listed in connection with Spinraza. One of them, U.S. patent 9,926,559 (the ‘559), will not expire until January 9, 2034, roughly 16 years after the expiration of the ‘197 patent.
Descovy is another similar example. According to the legacy Orange Book obtained by KEI via FOIA request, there have been eight patents listed in connection with this drug. U.S. patent 5,814,639 (the ‘639), which was assigned to Emory University and titled Method for the synthesis, compositions and use of 2′-deoxy-5-fluoro-3′-thiacytidine and related compounds, expired on March 29, 2016. However, the Orange Book currently also lists U.S. patent 9,296,769 (the ‘769 patent) for Descovy, assigned to Gilead Science. The ‘769 patent is set to expire on August 15, 2032 according to the Orange Book, which would be roughly 16 years after the ‘639 patent expired.
While it might not always be the case, the fact that some of these patents will expire long after the first one ever listed for the product does seem indicative of secondary patenting strategies. The public deserves to know that these products have been under some form of exclusivity for a very long time, and the Orange Book is an ideal research tool to provide this information.
Another way to potentially identify secondary patenting strategies is by counting the number of patents that have ever been listed in the Orange Book. To further illustrate this point we again used the legacy version of the Orange Book obtained by KEI via FOIA request, which has all patents listed from inception to August 2018. In that version of the Orange Book we found a total of 6,658 unique patents listed in connection to 1,767 applications. This represents an average of 3.76 patents per application. However, there are plenty of outliers. One drug product alone, Afrezza, a human recombinant insulin, listed a total of 44 unique patents. Imbruvica, a high priced drug used to treat B cell cancers, listed 27 unique patents in total.
It is relatively easy to figure out the number of patents that have ever been listed in the Orange Book using the historical data files. Academic researchers have used the legacy data files to draw this type of analysis in the past. Therefore we believe that implementing this recommendation does not require any changes in the format of the historical data files, other than publishing them periodically as we proposed in a previous section of these comments.
KEI asks that the FDA publish the number of patents ever listed for a specific product in the searchable electronic Orange Book database. The total number should include patents that have now expired and have been delisted from the current edition. This could be achieved by adding a statement in the entry for each product, along the line of “There have been N unique patents ever listed in the Orange Book for this product, and N of them are in the current edition.” The FDA should also add a search field for this data item, including filters. As such, a user seeking products that listed more than 20 patents in the Orange Book should be able to quickly find Afrezza, Imbruvica, and others in the same situation.
The FDA should expand the Orange Book to include data on the clinical trials that are cited by the FDA to support marketing approval of a product. The Orange Book already provides information on test data exclusivity, but does not provide information on the specific trials that benefit from such exclusive rights.
This Orange Book should report for each clinical trial used to obtain marketing approval, the trial identifier, such as the NCT number, as well as the trial phase, the patient enrollment, trial costs and information regarding the funding of the trials including the cost of the trial. The information on trial funding should include the total amount and percentage of costs paid the U.S. government, other governments, charities, and industry.
To facilitate the dissemination of this data, and arguably for other reasons, the FDA should require the medical reviews to use a standardized form to report the required meta data for the NCT number, trial type and phase and patient enrollment, for each trial submitted to the FDA to support marketing approvals.
Information on trial costs should be required by the FDA during the approval process, including the cost of the trial and the amount and percentage of costs paid the U.S. government, other governments, charities, and industry.
The Drug Trials Snapshots web page is a useful initiative, but it could be even more useful.
Trials Snapshots should include products approved before the program was launched in 2015, and include also biologics, vaccines, and gene and cell therapies, and information on who financed the trials.
The Orange Book should have a separate statement on the trials used to justify a pediatric extension, including the enrollment and costs of each trial, as well as information on who funded or subsidized the trials.
KEI reminds the FDA that the Seventy-second World Health Assembly adopted “WHA72.8, Improving the transparency of markets for medicines, vaccines, and other health products,” which included the following statements:
“Recognizing also that improving access to health products is a multidimensional challenge that requires action across, and adequate knowledge of, the entire value chain and life cycle, from research and development to quality assurance, regulatory capacity, supply chain management and use;
. ..
Noting the importance of both public- and private-sector funding for research and development of health products, and seeking to improve the transparency of such funding across the value chain;”
. . .
Agreeing that policies that influence the pricing of health products and that reduce barriers to access can be better formulated and evaluated when there are reliable, comparable, transparent and sufficiently detailed data2 across the value chain,
The proposals intended to improve transparency throughout the Orange Book should also be applied to the Purple Book. KEI filed comments relating to the enhancement of the Purple Book on May 4, 2020. We also incorporate those comments by reference to this submission. The fact that the U.S. has a two-tiered standard of patent landscape transparency for drugs and biologics is unfortunate, particularly since patent landscapes for biologic products are often more complex, and biosimilar competition is far less robust.
Part of the social bargain for a patent is to induce disclosure of know-how in return for a temporary monopoly. The Purple Book system deliberately undermines the usefulness of the disclosure, and contributes to considerable delays in entry by competitors, and makes biosimilar products less safe.
For each product protected by patents or a regulatory exclusivity the FDA should require applicants to provide periodic information about the number of units sold and the sales revenue. This information should be provided for each market, or at least be broken down by the United States and appropriate regions, and updated regularly, for example, by quarter or year. Companies already provide information on revenue by product and in some cases by geographic areas, to shareholders, for products that have a material impact on share prices.
The obligation to report units sold and sales revenue could be eliminated three years after all exclusivities expire.
Such disclosures are required by “WHA72.8 Improving the transparency of markets for medicines, vaccines, and other health products,” adopted at the 72nd World Health Assembly with enthusiastic support from the United States government. Operative paragraph 1(3) of that resolution urged WHO Member States to:
“to work collaboratively to improve the reporting of information by suppliers on registered health products, such as reports on sales revenues, prices, units sold, marketing costs, and subsidies and incentives;”
KEI asks that the FDA require applicants to disclose the license on patents, know-how, data or biologic resources that were needed to make, import or sell drugs, biologics, and cell and gene therapies, including the name of the parties; a description of the technology, materials, or data licensed; the field of use; geographic areas; the term; and the economic considerations.
Data about licensing profiles is important to understanding current and previous patent landscapes, as well as upstream efforts that led to drug approvals.
Policy experts, academics, advocates and the public in general deserve to know where the inventions related to approved drug products were initially conceived, who funded the research, and how much the licensees had to pay to acquire patents, know-how, data or biologic resources.