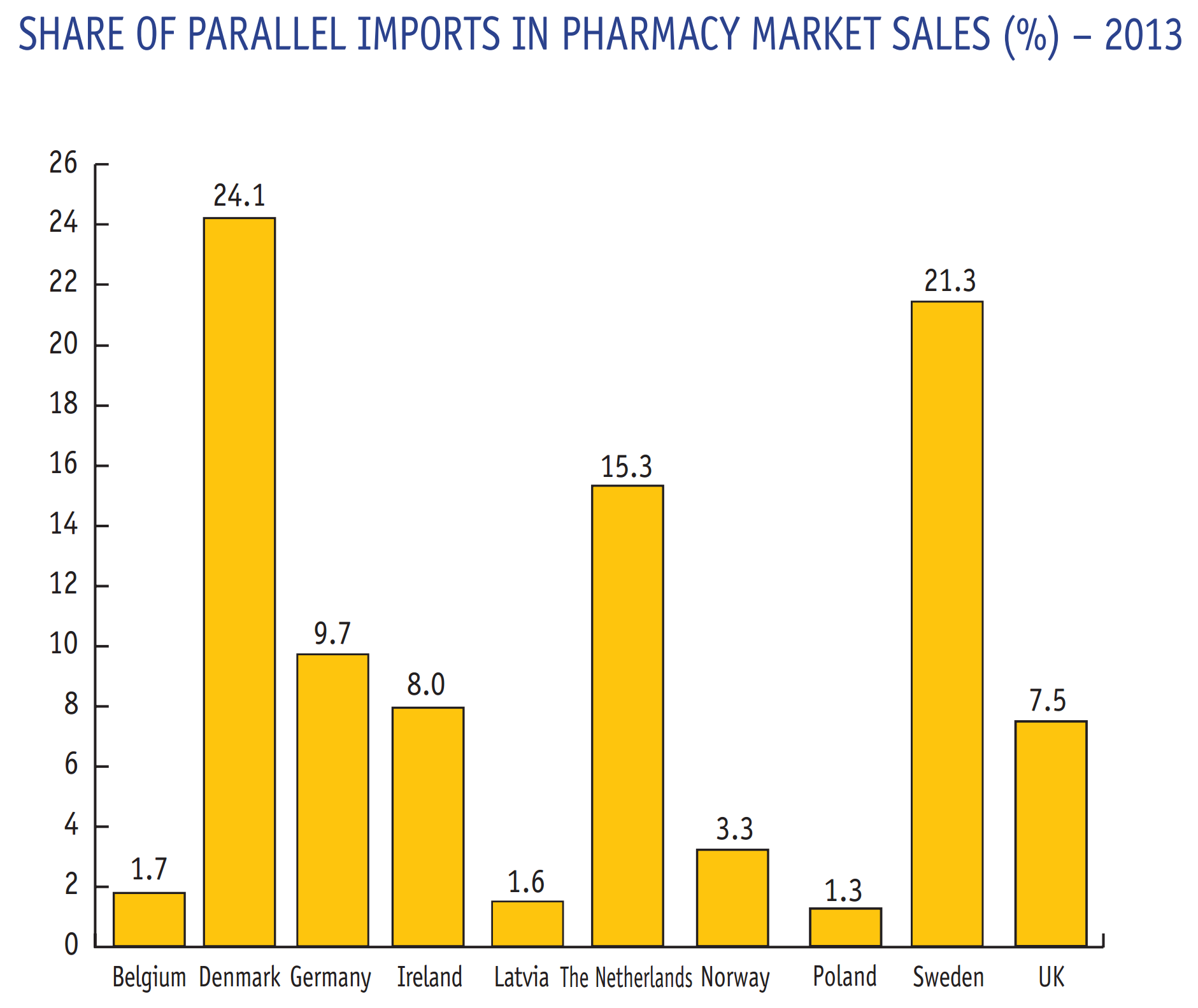
European parallel trade in pharmaceuticals, 2013
As candidates consider parallel trade in pharmaceutical drugs, a practice blocked by Obama during his presidency, here is how common it is in Europe, where it is regulated and mainstream, as reported in the EFPIA publication, the Pharmaceutical Industry in Figures. Key data, 2015.