On July 9, 2021, the Biden White House has issued an Executive Order on competition which includes opposition to the Trump effort to narrow Bayh-Dole march-in rights over pricing concerns. This is the first time since 1995 there has been… Continue Reading →
New: Biden White House Executive Order opposes change in Bayh-Dole March-In regulations. (KEI has an email list to discuss this issue here: http://lists.keionline.org/mailman/listinfo/bayh-dole-regulations_lists.keionline.org) The Department of Commerce’s National Institute of Standards and Technology (NIST) published on January 4, 2021, a… Continue Reading →
Other Transaction Agreements: Government Contracts that May Eliminate Protections for the Public on Pricing, Access and Competition, Including in Connection with COVID-19 KEI Briefing Note 2020:3 June 29, 2020 (revision of June 24 version) Kathryn Ardizzone James Love The most… Continue Reading →
Ventana Medical Systems, Inc., march-in request 1999. February 10. Jonathan Cohen, Director of Intellectual Property for Ventana Medical Systems, Inc., sends letter to Paul A. Gottlieb, Esq., Assistant General Counsel for Technology Transfer and Intellectual Property at the U.S. Department… Continue Reading →
For a general timeline of the Bayh-Dole Act, see this page. 1980. December 12. The Bayh-Dole Act was enacted into law as part of Public Law 96-517, including Section 203 that provide march-in rights. 1984. November 8. The Bayh-Dole statute was… Continue Reading →
Today KEI asked the Department of Commerce National Institute of Standards (NIST) to publish a notice in the Federal Register inviting comments on their Special Publication 1234, Draft Green Paper on Return on Public Investment, with an extended deadline. The… Continue Reading →
KEI filed comments on the NIST Draft Green paper on the return from investment on federally funded R&D (See: https://www.keionline.org/29518). We expect the comment period to be extended, but filed this on January 9, 2018, as an initial submission. KEI-comments-NIST-SP-1234-ROI-9Jan2018… Continue Reading →
Bayh-Dole cases involving royalty free or march-in rights 1997 Cellpro case This was a strong case involving two competing medical devices, both invented on NIH grants, and a bad ending. The NIH rejection of the Cellpro march-in request led to… Continue Reading →
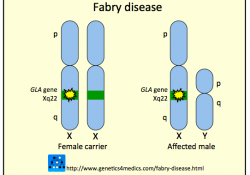
The 2010 Fabrazyme NIH Bayh-Dole march-in case https://www.keionline.org/cl/bayh-dole/fabrazyme The 2014 FTC complaint regarding collusion between Shire and Sanofi. https://www.keionline.org/22538