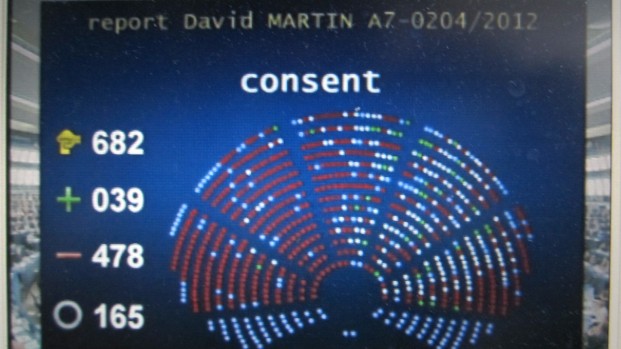
Video Interviews and Press Coverage from SCCR 24
The following interviews were recorded during the 24th meeting of the World Intellectual Property Organization (WIPO) Standing Committee on Copyright and Related Rights (SCCR). Most of the interviews are focused on the negotiations on a new WIPO treaty for persons who are blind or have other disabilities. The time of the videos varies from 16 seconds to more than 18 minutes. They are organized by the type of stakeholder, and the date of the interviews. This page will be updated during the meeting as I add more videos. Continue Reading