On Tuesday April 28, 2020, KEI submitted comments to the National Institutes of Health (NIH) regarding the “Prospective Grant of an Exclusive Patent License: AAV Mediated Exendin-4 Gene Transfer to Salivary Glands To Protect Subjects From Diabetes or Obesity” (85… Continue Reading →
On Friday, 24 April 2020, Knowledge Ecology International submitted comments on the resolution WHA73: “Covid-19 Response” proposed by the European Union (EU). The original zero draft text and the note verbale proposed by the European Union can be found here.… Continue Reading →
Under the notification of laws and regulations obligations of Article 63.2 of the World Trade Organization’s (WTO) TRIPS Agreement, on 23 April 2020, Canada sent a notification (IP/N/1/CAN/30) to the WTO in relation to “Bill C-13 An Act respecting certain… Continue Reading →
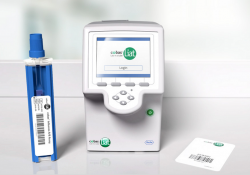
KEI Research Note 2020:1 Government funding related to the cobas Liat Analyzer PCR testing device. Luis Gil Abinader, April 22, 2020. Introduction Polymerase chain reaction (PCR) testing technique Automatization of the PCR testing technique SARS-Cov-2 emergency use authorizations for PCR… Continue Reading →
On Friday April 17, 2020, Knowledge Ecology International (KEI) submitted comments to the National Institutes of Health (NIH) regarding the “Prospective Grant of an Exclusive Patent License: The Development of Bispecific Antibodies Targeting Glypican 1 (GPC1) for the Treatment of… Continue Reading →
On Friday April 17, 2020, Knowledge Ecology International (KEI) submitted comments to the National Institutes of Health (NIH) regarding the “Prospective Grant of an Exclusive Patent License: Methods and Compositions for Adoptive Cell Therapy” to Lyell Immunopharma, Inc. (85 FR… Continue Reading →
On Wednesday, 15 April 2020, the European Union submitted a zero draft of a resolution for the World Health Assembly entitled WHA73: “Covid-19 Response”; this text was accompanied by a note verbale. The note verbale stated the following: We understand… Continue Reading →
Brazil-CL-Pandemics-KEI-10April2020 April 10, 2020 Members of the Brazil Congress It is our understanding that some large drug companies have raised objections to a new compulsory licensing authority for Brazil relating to pandemics. I would like to address the several objections… Continue Reading →
Background In 2001, the World Trade Organization (WTO) began negotiations on the rules regarding patents and access to medicine. While several issues were clarified and resolved in the November 2001 “Doha Declaration on TRIPS and Public Health”, the negotiations took… Continue Reading →
On April 6, 2020, the Director-General of the World Health Organization (WHO), Dr. Tedros Ghebreyesus, gave a media briefing on COVID-19 and the WHO’s efforts to combat the pandemic. In his remarks, Dr. Tedros specifically highlighted the proposal by the… Continue Reading →