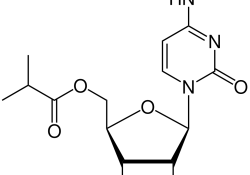
Amicus Brief of Professor Brook Baker on the human right principles and TRIPS-compliant interpretation of the compulsory license petition filed by KEI in the Dominican Republic
Last week, Professor Brook Baker filed an Amicus Brief responding to four of the arguments made by Pfizer against the compulsory license requested by KEI in the Dominican Republic to allow the distribution of the generic version of Paxlovid. His… Continue Reading