Access to Medicine
WHO Cancer Report – Key findings
On 18 December 2018, the World Health Organization (WHO) published a “Technical report on Pricing of cancer medicines and its impacts” (hereinafter referred to as the “WHO Cancer Report”). The mandate for the WHO Cancer Report emanates from operative paragraph… Continue Reading
2019: Joint Comments on NIH Prospective License to Medigen on RSV and Parainfluenza Vaccines (and NIH Response)
(UPDATE: The NIH provided a response to our comments on February 4, 2019) On January 7, 2019, KEI and MSF (Doctors Without Borders/Médecins Sans Frontières USA) submitted comments to the Federal Register notice (83 FR 65696) on the “Prospective Grant… Continue Reading
2019: Joint Comments on NIH Prospective License to Sixfold Biosciences on Nanoparticles Technology (and NIH Response)
(UPDATE: The NIH provided a response to our comments on January 11, 2019) On Monday January 7, 2019, KEI submitted joint comments on behalf of Health GAP, Social Security Works, the Union for Affordable Cancer Treatment, and Professor Brook Baker… Continue Reading
KEI Comments on Medicare Programs: International Pricing Index Model for Medicare Part B Drugs (CMS-5528-ANPRM)
KEI Comments on Medicare Programs: International Pricing Index Model for Medicare Part B Drugs (CMS-5528-ANPRM) Knowledge Ecology International (KEI) is a non-profit organization that focuses on the management of knowledge goods, including new drugs, vaccines and other medical therapies. Much… Continue Reading
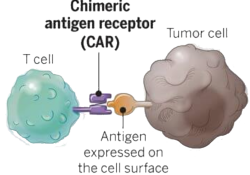
2018: NIH Prospective Grant of an Exclusive Patent License to ElevateBio for CAR T cancer treatment
The following are comments by KEI, UACT, Social Security Works, HealthGap, Public Citizen, Dr. Ophira Ginsbug and James Love on an NIH proposed exclusive license for patents on a CAR T technology for “Treatment of FMS-Like Tyrosine Kinase 3 (FLT3)… Continue Reading
SCP29: General statement of Knowledge Ecology International
On Monday, 3 December 2018, Knowledge Ecology International (KEI) delivered this opening statement at WIPO’s 29th Session of the Standing Committee on the Law of Patents (SCP). Thank you Chair. KEI welcomes the conclusions of SCP 28 which call upon… Continue Reading
NIH Preparing to License CAR Treatment Patents to Investors Behind Sovaldi and Soliris
In a recent Federal Register notice, the National Institutes of Health (NIH) has announced its intent to grant an exclusive patent license on chimeric antigen receptor (CAR) therapies for the treatment of certain cancers to ElevateBio, a company located in… Continue Reading
WIPO side event, December 6, 2018, on the Implications of Article 27.3(a) of the WTO TRIPS Agreement on the patentability of gene and cell-based therapies
DATE: Thursday, 6 December 2018 TIME: 1:30 PM to 3 PM VENUE: Room B CONVENER: Knowledge Ecology International (KEI) Knowledge Ecology International (KEI) cordially invites you to attend our side event during negotiations of the 29th Session of the Standing… Continue Reading
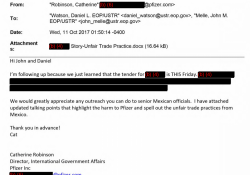
Pfizer asked USTR to intervene in Mexico, to remove a competing product from a tender in 2017
This October 11, 2017 email, from Catherine Robinson of Pfizer to Daniel Watson and John Meile of USTR, asks USTR to block a competitor from a tender to sell a drug. USTR provides one email and redacts details including the… Continue Reading