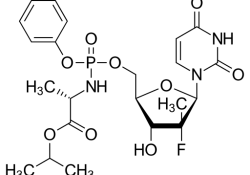
In the attached letter dated March 14, 2018 to Secretary of the Department of Health and Human Services (DHHS) Alex Azar, KEI is asking for an investigation into a failure to report NIH funding on US patent 7,964,580. This is… Continue Reading →
FOR IMMEDIATE RELEASE March 12, 2018 kim.treanor@keionline.org (202)-332-2670 A coalition of 16 advocacy organizations in the United States and Korea asked trade negotiators in the U.S. and Korea to reject the demands of the pharmaceutical industry, protect human rights, and… Continue Reading →
In a resolution dated the 9th of March, 2018, Minister Carmen Castillo Taucher of Chile’s Ministry of Health has announced that there are sufficient public health reasons to support a compulsory license on medicines for the hepatitis C virus (HCV),… Continue Reading →
On March 8, 2018, USTR and the interagency committee on the Special 301 held a hearing. KEI was one of the groups testifying. More on this process here: https://keionline.org/ustr/special301 I began our oral testimony discussing President Trump’s promise, during the… Continue Reading →
This is an update to a 2007 report regarding a district court decision in the Innogenetics v. Abbott case here, regarding Abbott’s infringement of Innogenetics’s patents for HCV genotyping. The previous blog discusses the ruling at the district court level… Continue Reading →
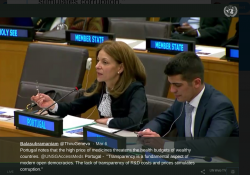
On Tuesday, 6 March 2018, Cristina Pucarinho, the Deputy Permanent Representative of Portugal to the United Nations made the following intervention at a UN briefing on Promoting Innovation and Access to Health Technologies in the Context of the Agenda 2030… Continue Reading →
World Health Assembly, May 2001 Statements May 21, 2001. Health GAP Report on the 54th World Health Assembly. May 18, 2001. CPT/Essential Action Comments on the World Health Assembly. May 17, 2001. HealthGAP Coalition Release on the World Health Assembly.… Continue Reading →
2018 Seventy-first World Health Assembly (WHA 71) 142nd WHO Executive Board Meeting (EB 142) 2017 Seventieth World Health Assembly (WHA 70) 2016 Sixty-ninth World Health Assembly (WHA 69) 2015 WHA 68 2014 WHA 67 2013 WHA 66 2012 WHA 65… Continue Reading →
KEI page on Research and Development Cost Transparency Legislation EB142: KEI statement on Addressing the global shortage of, and access to, medicines and vaccines, January 24th, 2018 Expert Panel charts a road map for WHO’s engagement on transparency and R&D,… Continue Reading →
On Tuesday, 27 February 2018, the representative of the Republic of South Africa delivered the following statement at the World Trade Organization’s TRIPS Council; South Africa delivered this statement under the agenda item on IP and the Public Interest: Regulatory… Continue Reading →