(Update: On November 4, 2021, the NIH provided a response to our comments on the license.) On October 12, 2021, Knowledge Ecology International (KEI) submitted comments to the National Institutes of Health (NIH) regarding the ““Prospective Grant of an Exclusive… Continue Reading →
(Update: The NIH provided responses to our comments on April 5, 2021 – 86 FR 10092 Response and 86 FR 10081 Response) On Friday March 5, 2021, Knowledge Ecology International (KEI) submitted comments to the National Institute of Health (NIH)… Continue Reading →
On 30 July 2020, the World Trade Organization (WTO) convened a formal meeting of the TRIPS Council. The TRIPS Council held discussions on two COVID-19 related topics: agenda item 3 on IP Measures in the Context of COVID-19 and agenda… Continue Reading →
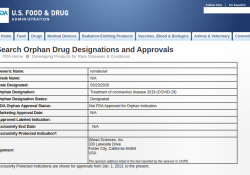
On Monday March 23, 2020, Gilead Sciences’ remdesivir received orphan designation from the US Food and Drug Administration (FDA) for the treatment of COVID-19. Remdesivir has been reported to be one of the candidates to potentially treat COVID-19, which the… Continue Reading →
Today the FDA granted Gilead Orphan Drug status for remdesivir for the treatment of COVID-19, on grounds this is a rare disease. The morning of the designation, the U.S. had confirmed, through testing, more than 35 thousand cases, including 8,477… Continue Reading →
KEI Briefing Note 2020:1: Role of the U.S. Federal Government in the Development of GS-5734/Remdesivir Kathryn Ardizzone. March 20, 2020. (Updated May 28, 2020) KEI-Briefing-Note-2020_1GS-5734-Remdesivir
(UPDATE: The NIH provided responses to our comments on August 14, 2019: 84 FR 33272 Response and 84 FR 33270 Response) On Monday July 29, 2019, Knowledge Ecology International (KEI) submitted joint comments to the NIH on behalf of KEI,… Continue Reading →
At today’s House Oversight Hearing on PrEP, one of issues will be access to Truvada (FTC+TDF), but also Descovy (FEMTRICITABINE+TENOFOVIR ALAFENAMIDE), a two drug combination, that uses TAF instead of TDF, and is less toxic than Truvada. The Goodrx price… Continue Reading →
In an order dated April 11, 2019, Judge Peter J. Messitte granted the NIH’s motion to dismiss for lack of jurisdiction in the lawsuit filed by KEI, which appealed an NIH decision to grant an exclusive license on a CAR… Continue Reading →
The attached document is a 78 page PDF file obtained from USTR under the Freedom of Information Act (copy here), regarding communications between Gilead and USTR, over Malaysia’s decision to grant a compulsory license on patents for HCV treatments. This… Continue Reading →