Livestream here: https://www.youtube.com/watch?v=iNMpuQfH088 On 14 May 2020, Health Action International, Knowledge Ecology International, Medicines Law & Policy, Pharmaceutical Accountability Foundation, and Wemos will convene a briefing entitled: “The WHO Covid-19 Technology Pool: The solution to ensure global access to Covid-19… Continue Reading →
KEI Briefing Note 2020:2. The Federal Government’s Authority to Restrict or Eliminate Contractors’ Rights to Federally-Funded Inventions in “Exceptional Circumstances” Kathryn Ardizzone, May 1, 2020. KEI_Briefing_Note_2020_2_Exceptional_Circumstances
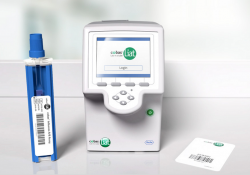
KEI Research Note 2020:1 Government funding related to the cobas Liat Analyzer PCR testing device. Luis Gil Abinader, April 22, 2020. Introduction Polymerase chain reaction (PCR) testing technique Automatization of the PCR testing technique SARS-Cov-2 emergency use authorizations for PCR… Continue Reading →
On April 6, 2020, the Director-General of the World Health Organization (WHO), Dr. Tedros Ghebreyesus, gave a media briefing on COVID-19 and the WHO’s efforts to combat the pandemic. In his remarks, Dr. Tedros specifically highlighted the proposal by the… Continue Reading →
In Canada, the foreground intellectual property arising under procurement contracts by default belongs to the contractor. However, there are several exceptions to this rule. One key policy provision allows the government to claim title of the foreground intellectual property “where…… Continue Reading →
The following is a letter that KEI and Public Citizen sent to Congressional leadership on March 26, 2020, supporting the proposal by the President of Costa Rica to the World Health Organization to create a pool for rights in technology… Continue Reading →
March 27, 2020. We are writing to ask the WHO and its Member States to support the proposal by Costa Rica for the creation of a global pooling mechanism for rights in the data, knowledge and technologies useful in the… Continue Reading →
A House coronavirus stimulus bill contains a provision concerning the use of Other Transactions Authority to fund COVID-19 diagnostics. The provision appears to authorize the Department of Homeland Security (DHS) to award $2.2 billion to private sector companies to develop… Continue Reading →
(KEI blogs and other work on COVID-19 are here: https://www.keionline.org/coronavirus) A letter from Costa Rica, signed by Carlos Alarado Quesada, the President, and Dr. Daniel Salas Peraza, the Minister of Health, to Dr. Tedros Adhanom Ghebreyesus, was sent this evening… Continue Reading →
KEI Briefing Note 2020:1: Role of the U.S. Federal Government in the Development of GS-5734/Remdesivir Kathryn Ardizzone. March 20, 2020. (Updated May 28, 2020) KEI-Briefing-Note-2020_1GS-5734-Remdesivir