On October 14, 2025, Knowledge Ecology International (KEI) provided the NIH with comments regarding the “Prospective Grant of Exclusive License, Inter-Institutional Agreement-Institution Lead: Development of Zika Virus Strains for Use in Oncolytic Therapy” (90 FR 46414) to Advocate Aurora Research… Continue Reading →
On Monday, 3 November 2025, Knowledge Ecology International (KEI) delivered the following intervention at the Third meeting of the open-ended Intergovernmental Working Group on the WHO Pandemic Agreement. Given the significant attacks on the WHO and the importance of restoring… Continue Reading →
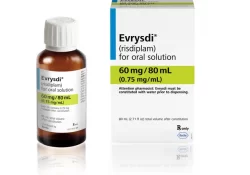
Below is a short note on good news on a court case in India concerning access to risdiplam, a treatment for spinal muscular atrophy (SMA). The note is followed by a statement on the case by Third World Network (TWN),… Continue Reading →
IGC Statement by KEI at 2025 WIPO GA Reflecting on the fact that the 2024 Diplomatic Conference on the treaty to disclose genetic resources and associated traditional knowledge came after more than two decades and more than 50 meetings of… Continue Reading →
On Tuesday afternoon, 24 June 2025, the Core Group (Bangladesh, Brazil, China, Egypt, India, Indonesia, Senegal, South Africa, Thailand) convened an informal consultation to consider the revised version draft resolution, “Access to medicines, vaccines and other health products in the… Continue Reading →
The World Intellectual Property Organization (WIPO) convened the 39th session of its Program and Budget Committee (PBC) from 16 June 2025 to 20 June 2025. With respect to WIPO’s proposed Program and Budget for 2026/2027, the PBC passed the following… Continue Reading →
On Friday, 13 June 2025, a bloc of countries known as the the Core Group (Brazil, China, Egypt, Indonesia, India, Senegal, South Africa and Thailand) circulated a draft resolution on “Access to medicines, vaccines and other health products in the… Continue Reading →
This is a PDF copy of the letter June 13, 2025 Robert F. Kennedy, Jr. Secretary U.S. Department of Health and Human Services 200 Independence Ave SW, Washington, DC 20201 RE: Request for issuance of authorization and consent to use… Continue Reading →
CONFIDENTIAL RELEASED IN FULL CONFIDENTIAL PTQ1906 PAGE 01 GENEVA 03470 01 OF 09 271621Z ACTION 10-00 425261 271705Z /38 O 271615Z MAY 98 FM USMISSION GENEVA TO SECSTATE WASHDC IMMEDIATE 6208 INFO IO COLLECTIVE AMEMBASSY LONDON AMEMBASSY BRASILIA AMEMBASSY BUENOS… Continue Reading →
As mentioned in a previous blog, Under the topic of industrial designs, the 48th session of the WIPO Standing Committee on the Law of Trademarks, Industrial Designs and Geographical Indications (SCT) is considering two submissions. The first submission is an… Continue Reading →