30 April 1997 USTR REPORT ON SPECIAL 301 ANNUAL REVIEW FACT SHEET “SPECIAL 301” ON INTELLECTUAL PROPERTY RIGHTS ACTIONS TAKEN Acting United States Trade Representative Charlene Barshefsky today announced the Administration’s decision with respect to this year’s review under the… Continue Reading →
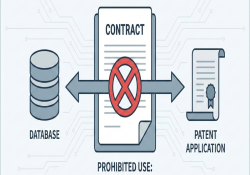
Contracts that restrict patent filing or impose patent-licensing obligations This briefing note illustrates examples of contractual language from data-sharing agreements and material transfer agreements that limit patent filing or impose specific licensing terms. KEI Briefing Note 2025:4 Arianna Schouten December… Continue Reading →
On Tuesday afternoon, 2 December 2025, Knowledge Ecology International made the following statement on copyright limitations and exceptions at the 47th session of the WIPO Standing Committee on Copyright and Related Rights. I wanted to comment first on the African… Continue Reading →
Below is a short note on good news on a court case in India concerning access to risdiplam, a treatment for spinal muscular atrophy (SMA). The note is followed by a statement on the case by Third World Network (TWN),… Continue Reading →
On 20 May 2025, the World Health Assembly (WHA) adopted the WHO Pandemic Agreement “pursuant to Article 19 of the Constitution of the World Health Organization” through resolution WHA78.1. While achieving consensus on a number of fronts including pandemic prevention… Continue Reading →
On July 28, 2025, Knowledge Ecology International (KEI) submitted a Freedom of Information Act request for records concerning the Food and Drug Administration (FDA) Orphan Products Grant Program. The program (administered by the FDA’s Office of Orphan Products Development (OOPD))… Continue Reading →
On Thursday afternoon, 10 July 2025, Knowledge Ecology Interational (KEI) delivered the following statement on agenda item 12(ii), Report from the Standing Committee on the Law of Patents (SCP). KEI statement on the Standing Committee on the Law of Patents… Continue Reading →
KEI has filed two comments with USTR in its “Request for Comments Regarding Foreign Nations Freeloading on American-Financed Innovation,” (90 FR 23105). This comment summarized some of KEI’s past comments on the trade related aspects of biomedical R&D and public… Continue Reading →
On Tuesday afternoon, 24 June 2025, the Core Group (Bangladesh, Brazil, China, Egypt, India, Indonesia, Senegal, South Africa, Thailand) convened an informal consultation to consider the revised version draft resolution, “Access to medicines, vaccines and other health products in the… Continue Reading →
The World Intellectual Property Organization (WIPO) convened the 39th session of its Program and Budget Committee (PBC) from 16 June 2025 to 20 June 2025. With respect to WIPO’s proposed Program and Budget for 2026/2027, the PBC passed the following… Continue Reading →