SCCR24: Opening statement of the African Group
The World Intellectual Property Organization (WIPO) has convened the 24th Standing Committee on Copyright and Related Rights (SCCR) which will take place from 16 July 2012 to 25 July 2012 at its headquarters in Geneva, Switzerland. Ambassador Darlington Mwape (Zambia) is chairing the 24th SCCR. The morning session of the SCCR witnessed opening remarks by group coordinators. The following statement was delivered by Egypt on behalf of the African Group.
Posner’s dismissals of the patent infringement suits in Apple versus Motorola cites eBay and compulsory licensing
On 22 June 2012, Judge Richard Posner, dismissed with prejudice the patent infringement suits filed in Apple Inc. and NEXT Software, Inc. v Motorola, Inc. and Motorola Mobility, Inc. in the United States District Court for the Northern District of Illinois (Eastern Division). In this case, the judge cited the eBay decision noting that neither party was entitled to injunctive relief as neither party demonstrated that “damages would not be an adequate remedy”. Continue Reading
KEI notes on the 13th round of TPPA negotiations in San Diego, CA
The 13th round of negotiations for the Trans-Pacific Partnership Agreement (TPPA) took place in San Diego, CA, USA from 2-10 July 2012.
Notes on Intellectual Property
Leahy on patent trolls, licensing on reasonable terms
In a May 9, 2012 hearing in the Senate Judiciary Committee on oversight of the intellectual property enforcement coordinator, Senator Leahy asked Victoria Espinel about patent trolls, and failures to license patents on reasonable terms to achieve interoperability where standards are important. Leahy asked Victoria Espinel to work with USDOJ’s antitrust officials to deal with abuses by patent holders in both cases.
1 KEI Files Brief in Kirtsaeng v John Wiley & Sons (copyright first sale doctrine case)
Today, July 9, 2012, Knowledge Ecology International (KEI) filed an amicus brief in the Supreme Court of the United States in support of neither party in the case Kirtsaeng v. John Wiley & Sons.
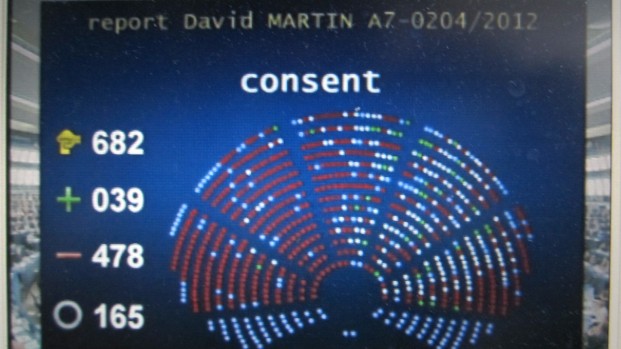
Continue Reading 1 European Parliament votes 478 to 39 to reject ACTA
The vote to reject a delay of the final ACTA vote was 420 Vs 255, followed by the rejection of ACTA by a vote of 478 to 39.
KEI Director James Love statement:
Continue ReadingJoint Statement of Civil Society Groups on U.S. TPP Copyright Proposal
The below is a joint statement from EFF, Knowledge Ecology International, Public Knowledge, and Public Citizen.
Joint Statement of Civil Society Groups on U.S. TPP Copyright Proposal
USPTO “clarifies” June 27, 2012 testimony on biologics exclusivity and India compulsory license
Deputy USPTO Director Teresa Stanek Rea has issued a retraction of her statement regarding Administration support for 12 years of exclusive rights in test data for biologic drugs, and moderated somewhat her statement on the India compulsory license for Nexavar. USTR also issued a statement on the issue of biologic test data in response to Rea’s earlier comments.
2 Copyright Limitations and Exceptions: What does the secret TPPA text say?
US Congress
Hearings on intellectual property rights, 112th Congress
House of Representatives
- April 26, 2012. Hearing on: “International Patent Issues: Promoting a Level Playing Field for American Industry Abroad” Subcommittee on Intellectual Property, Competition and the Internet. http://judiciary.edgeboss.net/wmedia/judiciary/ip/ip04262012.wvx
- May 26, 2012. Hearing on: Implementation of the Leahy-Smith America Invents Act, Full Judiciary Committee Hearing. http://judiciary.edgeboss.net/wmedia/judiciary/full/fullcomm05162012.wvx