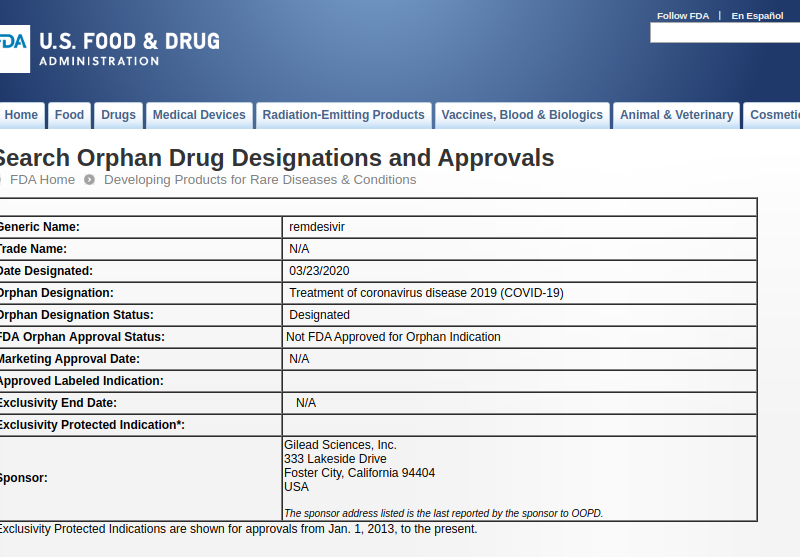
Today the FDA granted Gilead Orphan Drug status for remdesivir for the treatment of COVID-19, on grounds this is a rare disease. The morning of the designation, the U.S. had confirmed, through testing, more than 35 thousand cases, including 8,477 cases in the past 24 hours. Everyone wishes this was a rare disease, but unfortunately, it isn’t, and the testing will certainly confirm that shortly.
This is the U.S. Orphan Drug Act statutory definition for a rare disease.
-
DESIGNATION OF DRUGS FOR RARE DISEASES OR CONDITIONS
SEC. 526 OF THE FEDERAL FOOD, DRUG, AND COSMETIC ACT [21 USC 360bb].
(2) For purposes of paragraph (1), the term “rare disease or condition” means any disease or condition which
(A) affects less than 200,000 persons in the United States, or
(B) affects more than 200,000 in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for such disease or condition will recovered from sales in the United States of such drug. Determinations under the preceding sentence with respect to any drug shall be made on the basis of the facts and circumstances as of the date the request for designation of the drug under this subsection is made.
So what just happened?
There are patents on remdesivir held by Gilead. But under 28 USC 1498(a), the US can overcome the patent monopoly, and buy a generic. It can contract with the generic company to make and import to the US. There is already at least one generic company that we have talked to that is developing a generic version. But to sell a drug in the United States, you need FDA approval.
The FDA is not allowed to register an generic version for use for COVID-19 for 7 years under the action the FDA took today, because the U.S. government did not include an exception to the regulatory monopoly in the Orphan Drug Act. Congress can and should fix this. There should not be an exception proof monopoly for rare diseases.
Also, the FDA should be challenged for even declaring COVID-19 a rare disease, given the expectations of how many people are already infected and how many will be infected in a few days.
One key phrase in the statue is this:
“Determinations under the preceding sentence with respect to any drug shall be made on the basis of the facts and circumstances as of the date the request for designation of the drug under this subsection is made.”
The terms “facts and circumstances” should include the evidence the virus was a risk to spread widely.
(More of coronavirus/COVID-19 here: https://keionline.org/coronavirus).