At the closing to the 13th WTO Ministerial in Abu Dhabi, the World Trade Organization adopted a decision to extend the moratorium on non-violation and situation complaints. The text of the decision (WT/MIN(24)/39) is reproduced here: The Ministerial Conference decides… Continue Reading →
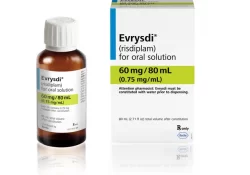
On March 1st, Knowledge Ecology International sent a letter to The Hon. Mark Holland, Ministry of Health, and The Hon. François-Philippe Champagne, Minister of Innovation, Science, and Industry, urging the prompt consideration of adding risdiplam, a drug to treat spinal… Continue Reading →
On 4 December 2023, the World Trade Organization (WTO) published a document (WT/GC/W/913, IP/C/W/694/Rev.1) entitled, “Decision text on extension of the 17 June 2022 Ministerial Decision to COVID-19 therapeutics and diagnostics”. This document is a communication from Bangaldesh, The Plurinational… Continue Reading →
On Monday, 27 November 2023, Brazil delivered the following general statement at WIPO’s Thirty-First Session of the Committee on Development and Intellectual Property. The statement was delivered by Ambassador Guilherme Patriota, one of the architects of WIPO’s Development Agenda. General… Continue Reading →
The USITC has issued a 497 page report on the WTO negotiations over TRIPS and COVID. https://www.usitc.gov/publications/332/pub5469.pdf The USITC release states: The U.S. International Trade Commission (USITC) today released a report about COVID-19 diagnostics and therapeutics and certain flexibilities under… Continue Reading →
Today, Knowledge Ecology International (KEI) submitted a letter to the Commissioner of the Food and Drug Administration (FDA) regarding the mismatch between the cost to perform pediatric extension trials and the cost to the public of the six month extension… Continue Reading →
In May 2023, the World Health Organization (WHO) published the global vaccine market report. Underpinning this technical report on vaccine market dynamics was a strong push by WHO’s Director-General, Tedros Adhanom Ghebreyesus, for increased transparency and equity; transparency is mentioned… Continue Reading →
Articles 2023, August 7. We Need Smart Intellectual Property Laws for Artificial Intelligence: “One-size-fits-all” regulation will sideline medical and research benefits promised by the advent of artificial intelligence. Article by James Love in Scientific American. New EU AI legislation Comments… Continue Reading →
Update: S.2333 passed Senate HELP today on a 17-3 bipartisan vote, with the delinkage study included. On Thursday, July 20, 2023, the Senate HELP Committee will consider a bill to reauthorize the Pandemic and All-Hazards Preparedness and Response Act (PAHPA).… Continue Reading →