On 18 December 2018, the World Health Organization (WHO) published a “Technical report on Pricing of cancer medicines and its impacts” (hereinafter referred to as the “WHO Cancer Report”). The mandate for the WHO Cancer Report emanates from operative paragraph… Continue Reading →
On July 18, 2018, the U.S. House Energy and Commerce committee gave its approval to HR 6378, the Pandemic and All-Hazards Preparedness Act, known as PAHPA. During the mark up, Reps. John Shimkus (R-IL) and Tony Cárdenas (D-CA) proposed attaching… Continue Reading →
On Monday, 9 July 2018, Knowledge Ecology International (KEI) made the following statement on exceptions and limitations to patent rights. KEI made the following proposal: In terms of future work on this item on exceptions and limitations to patent rights,… Continue Reading →
In May 2017, the 70th World Health Assembly passed a cancer resolution (WHA70.12, Cancer prevention and control in the context of an integrated approach). At the time KEI provided the following commentary on the cancer resolution: On Tuesday, May 30,… Continue Reading →
Add some hearings.. 1983 Oversight on implementation of the Orphan Drug Act (Public Law 97-414) : hearing before the Committee on Labor and Human Resources, United States Senate, Ninety-eighth Congress, first session, on reviewing the radioepidemiological tables and thyroid cancer… Continue Reading →
(More on this topic here: https://www.keionline.org/orphan-drugs and here.) 1980 April 17, 1980. Congresswoman Elizabeth Holtzman introduces H.R.7089 (96th Congress) – “A bill to establish an office in the National Institutes of Health to assist in the development of drugs for… Continue Reading →
Orphan Drug Timeline Data from Orphan Drug Tax Credit 2017. What does the Orphan Drug Tax Credit tell us about the Costs of Clinical Trials? Bill of Health. November 15. From 2010 to 2016, the average qualifying trial costs claimed… Continue Reading →
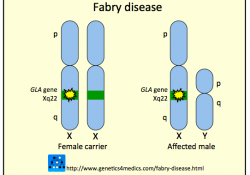
The 2010 Fabrazyme NIH Bayh-Dole march-in case https://www.keionline.org/cl/bayh-dole/fabrazyme The 2014 FTC complaint regarding collusion between Shire and Sanofi. https://www.keionline.org/22538
More on Fabry here: https://keionline.org/fabry NIH rejects Fabrazyme March-In Petition December 7, 2010. Press release from Fabry patients: DHHS denies patient’s march-in request to end Genzyme’s rationing of treatment for Fabry Disease citing that FDA rules block manufactures from supplying… Continue Reading →
Contact: Kim Treanor 202-332-2670; kim.treanor@keionline.org January 5, 2018 The National Institutes of Health (NIH) has proposed an exclusive license with Gilead for certain patent applications for inventions that target CD-30 proteins and CAR T technologies. The proposed license is to… Continue Reading →