Roche
Proposal for Addition Of Risdiplam to WHO Model List Of Essential Medicines, for the treatment of Spinal Muscular Atrophy (SMA).
On November 1, 2024, Knowledge Ecology International (KEI) submitted a proposal for the addition of risdiplam to the World Health Organization’s (WHO) Model List of Essential Medicine (EML). You can access the full proposal here: risdiplam2WHOEML1Nov2024 The EML, updated every… Continue Reading
KEI asks WHO to include risdiplam, treatment for Spinal Muscular Atrophy, on the Essential Medicines List (EML)
KEI letter to Roche requesting voluntary license to patents on risdiplam, a treatment for Spinal Muscular Atrophy (SMA)
This is a letter to Roche requesting a voluntary license to the patents on risdiplam, a treatment for Spinal Muscular Atrophy (SMA). KEI-Risdiplan-patent-license-request-8july2022 This was the August 5, 2022 response from Roche. Roche-Response-to-Risdiplam-license-request–5Aug2022 KEI wrote to Roche again on February… Continue Reading
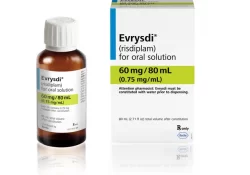
risdiplam
Risdiplam, originally developed under contract with the SMA Foundation by PTC Therapeutics and licensed to Roche for sale under the brand name Evrysdi, is a medication used to treat spinal muscular atrophy and the first oral medication approved to treat… Continue Reading
Access to SMA therapy: Iman’s Story
Recent years have seen several blockbuster treatments enter the market, many rooted in publicly funded foundational and pre-clinical research, and benefiting from government subsidies. In particular, we have conducted extensive research on Spinal Muscular Atrophy (SMA), which has regularly hit… Continue Reading
COVID-19 Contracts
United States of America Below are links to copies of the contracts entered into by the US government for technologies to combat the COVID-19 pandemic. KEI is updating this page as we gain access to more contracts, and less redacted… Continue Reading
KEI Research Note 2020:2 Role of Private Sector, Governments and Charities in Funding Research and Development Related to Tocilizumab
KEI Research Note 2020:2 Role of Private Sector, Governments and Charities in Funding Research and Development Related to Tocilizumab Luis Gil Abinader, May 28, 2020. From the Introduction: The funding of R&D for tocilizumab can be described as having three… Continue Reading
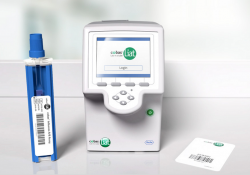
KEI Research Note 2020:1 Government funding related to the cobas Liat Analyzer PCR testing device
KEI Research Note 2020:1 Government funding related to the cobas Liat Analyzer PCR testing device. Luis Gil Abinader, April 22, 2020. Introduction Polymerase chain reaction (PCR) testing technique Automatization of the PCR testing technique SARS-Cov-2 emergency use authorizations for PCR… Continue Reading
Verinata v. Ariosa – compulsory license granted to Roche over prenatal screening technologies under eBay v. MercExchange
On July 19, 2018, the U.S. District Court, Northern District of California, denied a permanent injunction requested by Illumina, Inc, and instead ruled that a forward looking royalty for continued non-voluntary use of the invention, a type of compulsory license,… Continue Reading