On July 18, 2025, KEI submitted comments regarding the prospective exclusive licensing by the NIH of “The Development of an in vivo Anti-CD19 Chimeric Antigen Receptor (CAR) for the Treatment or Prevention of B Cell Mediated Autoimmune Diseases” (90 FR… Continue Reading →
On 14 May 2024, the Pharmaceutical Accountability Foundation (PAF) had their first hearing in their case against Abbvie at the Amsterdam District Court. The case concerns Abbvie’s pricing practices for its blockbuster drug Humira (adalimumab), one of the world’s best-selling… Continue Reading →
On October 2, 2023, Knowledge Ecology International submitted written comments to the Centers for Medicare & Medicaid Services (CMS) in conjunction with the Medicare Price Negotiation public consultation process. CMS is hosting a series of patient-focused listening sessions this fall,… Continue Reading →
KEI Notes on the Clinical Studies for Imbruvica (Ibrutinib) KEI Briefing Note 2023:3 October 2, 2023 James Love KEI-BN-2023-3
Notes on the Preclinical Development of Imbruvica (Ibrutinib) KEI Briefing Note 2023:4 October 2, 2023 Arianna Schouten KEI-BN-2023-4
For a general timeline of the Bayh-Dole Act, see this page. 1980. December 12. The Bayh-Dole Act was enacted into law as part of Public Law 96-517, including Section 203 that provide march-in rights. 1984. November 8. The Bayh-Dole statute was… Continue Reading →
The biologic drug, Humira (adalimumab) is currently the best selling drug in the world, with revenues of more than $16 billion in 2016, and $4.7 billion in the 2nd quarter of 2017 ($52 million per day). 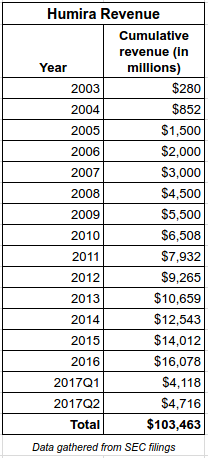
Continue Reading →
Attached is a letter sent on September 14, 2017 to Andrew Bremberg, an Assistant to the President and the Director of the Domestic Policy Council at the White House, and Keagan Lenihan, a Senior Adviser to HHS Secretary Tom Price, regarding Zinbrytra (INN: daclizumab), a drug to approved by the FDA to treat multiple sclerosis. (PDF version here)
Continue Reading →
On March 18, 2015, KEI, KEI Europe, and Essential Inventions submitted proposals for global voluntary licences for all patents necessary for hepatitis C (HCV) medicines to five drug companies — AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck.
Continue Reading →