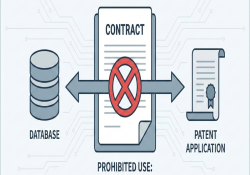
Contracts that restrict patent filing or impose patent-licensing obligations This briefing note illustrates examples of contractual language from data-sharing agreements and material transfer agreements that limit patent filing or impose specific licensing terms. KEI Briefing Note 2025:4 Arianna Schouten December… Continue Reading →
Note on Liability Rules KEI Briefing Note 2025:3 James Love, Thiru Balasubramaniam Draft, October 29, 2025 KEI-BN-2025-3
Selected Documents Prepared by the WIPO Secretariat for the Intergovernmental Committee (IGC) on Intellectual Property and Genetic Resources, Traditional Knowledge and Folklore IGC1 (April 2001) to IGC51 (June 2025) KEI Briefing Note: 2025:2 Arianna Schouten October 28, 2025 KEI-BN-2025-2
On October 2, 2023, Knowledge Ecology International submitted written comments to the Centers for Medicare & Medicaid Services (CMS) in conjunction with the Medicare Price Negotiation public consultation process. CMS is hosting a series of patient-focused listening sessions this fall,… Continue Reading →
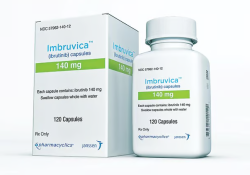
KEI Notes on the Clinical Studies for Imbruvica (Ibrutinib) KEI Briefing Note 2023:3 October 2, 2023 James Love KEI-BN-2023-3
Notes on the Preclinical Development of Imbruvica (Ibrutinib) KEI Briefing Note 2023:4 October 2, 2023 Arianna Schouten KEI-BN-2023-4
Mismatch between the cost to perform pediatric extension trials and the cost to the public of the six month extension of the drug monopoly KEI Briefing Note 2023:2 September 20, 2023 James Love, Manon Ress and Claire Cassedy KEI-BN-2023-2
The National Institute of Health (NIH) recently held an all-day workshop titled, “Transforming Discoveries Into Products: Maximizing NIH’s Levers to Catalyze Technology Transfer.” KEI Director James Love participated in the July 31st workshop, speaking on the panel on “How NIH… Continue Reading →
Canadian Experience with Compulsory Licensing under the Canadian Access to Medicines Regime KEI Briefing Note 2021:2 31 March 2021 Arianna Schouten PDF available here: KEI-Briefing-Note-2021-2-CAMR-Canadian-Compulsory-Licensing